Abstract
Introduction: The most common thromboembolic condition in coronavirus disease 2019 (COVID-19) is acute pulmonary embolism (APE). Factors associated with transfer to the intensive care unit among cases diagnosed with COVID-19 are separately known for COVID-19 and APE. However, it is important to identify factors associated with transfer to intensive care in patients with COVID-19-related APE. This study aimed to determine these factors in the coexistence of these conditions.
Methods: Adult patients diagnosed with APE by pulmonary computed tomography angiography were included in this cross-sectional study. The patients’ demographic and laboratory data, Wells scores, pulmonary embolism severity index (PESI) scores, and imaging findings were recorded. Pairwise comparisons were made between the patients with and without intensive care unit admissions.
Results: Of the 123 patients included in the study, 38 (30.8%) were transferred to the intensive care unit. In pairwise comparisons, age, number of comorbidities, lactate dehydrogenase, neutrophil-to-lymphocyte ratio, C-reactive protein (CRP), CRP-to-albumin ratio, d-dimer values, Wells scores, and PESI scores were higher among patients who transferred to intensive care. A higher Wells score (odds ratio (OR) = 1.70, 95% confidence interval (CI) =1.13 – 2.56, p=0.011), PESI score (OR = 1.03, 95% CI = 1.00 – 1.07, p=0.048), and CRP (OR = 1.01, 95% CI = 1.00 – 1.02, p=0.049) were associated with admission to the intensive care unit among patients with COVID-19-related APE.
Discussion and Conclusion: High Wells and PESI scores and CRP levels in adult patients hospitalized with a diagnosis of COVID-19-related APE were determined to increase the probability of transfer to the intensive care unit. Therefore, it is recommended to monitor these patients more closely for intensive care needs.
Keywords: critical care, pulmonary embolism, pulmonary embolism severity index, SARS-CoV-2 infection, Wells score
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Following its emergence, COVID-19 rapidly spread throughout the world, resulting in a pandemic (1). Thrombotic events may occur in patients with COVID-19 due to endothelial inflammation, complement activation, platelet activation, endothelial dysfunction, and immobilization. In addition to these risk factors, the activation of the coagulation system leads to a predisposition to thrombosis in both the venous and arterial systems. One of the most commonly detected thromboembolic events is acute pulmonary thromboembolism (APE) (2,3), which is among the conditions that result in the need for intensive care in cases of COVID-19.
It is important to determine factors associated with the development of intensive care needs in APE. Thus, patients need to be monitored in intensive care units, which increases the number of intensive care beds required as well as the morbidity and mortality associated with COVID-19 (2). Furthermore, although previous studies have examined factors associated with transfer to the intensive care unit separately in terms of APE and COVID-19, there is only limited research concerning factors associated with transfer to the intensive care unit in patients with COVID-19-related APE.
This study aimed to determine factors associated with transfer to the intensive care unit among hospitalized adult patients diagnosed with COVID-19-related APE.
Materials and Methods
Patients
This cross-sectional study was conducted at a tertiary hospital with approximately 3,100 inpatient beds and 700 intensive care beds in Ankara, Türkiye. The study included patients aged over 18 years who were hospitalized due to COVID-19 and were diagnosed with APE by pulmonary computed tomography (CT) angiography during their hospital stay, from April 1, 2020, through March 17, 2021. No exclusion criteria were applied.
Data and definitions
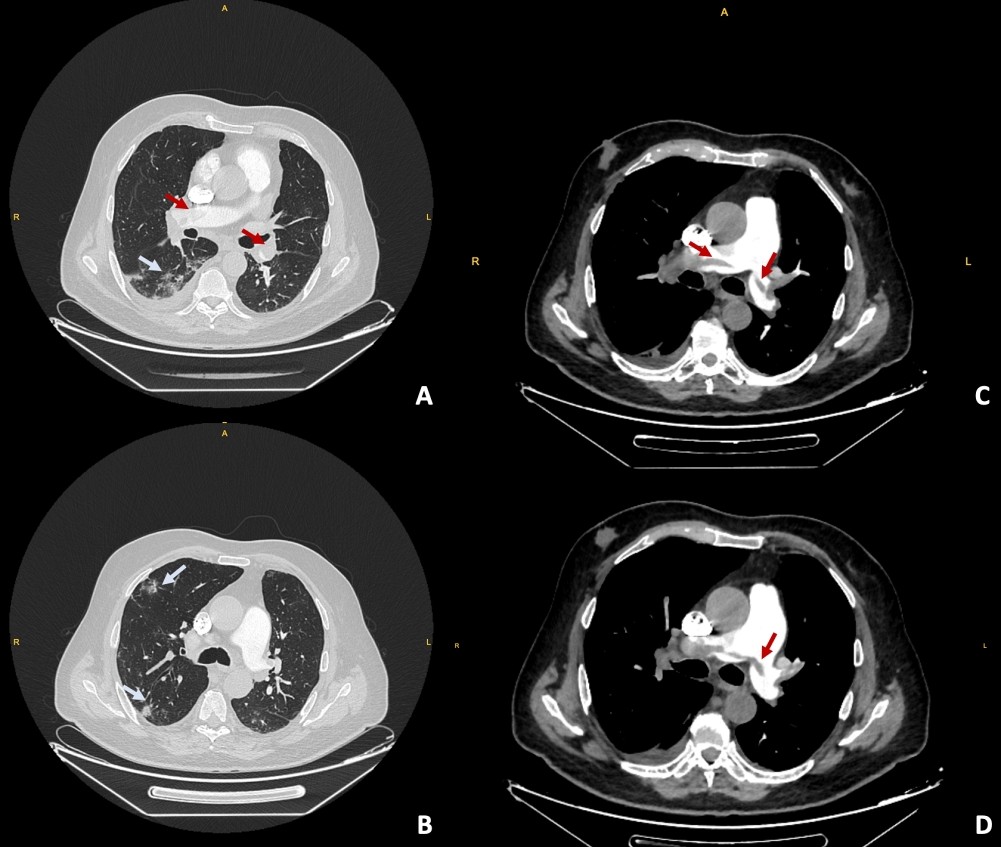
Data on gender, age, comorbidities, history of cancer, medication use, and length of hospital stay for all patients with COVID-19-related APE were obtained from the hospital’s electronic records. Laboratory tests were completed within 24 hours of diagnosis and included the complete blood count, neutrophil-to-lymphocyte ratio (NLR), serum albumin, creatinine, troponin I, brain natriuretic peptide, d-dimer, prothrombin time, activated partial thromboplastin time, lactate dehydrogenase (LDH), C-reactive protein (CRP), procalcitonin, ferritin, and interleukin-6 values. In addition, echocardiography (ejection fraction and pulmonary artery pressure), lower extremity Doppler ultrasonography, and CT pulmonary angiogram (CTPA) findings were recorded. The CRP-to-albumin ratio was obtained by dividing CRP in mg/dl and serum albumin in g/dl. The NLR was calculated by dividing the neutrophil count by the leukocyte count. The oxygen therapy received by the patients, the treatments applied for COVID-19, and the use of methylprednisolone at a dose of 250 mg/day and above were also noted. In-hospital mortality was evaluated for all participants. Patients with a COVID-19 diagnosis within 45 days before or 14 days after CTPA were considered to have COVID-19-related APE (4,5). The COVID-19 diagnosis was based on either a positive reverse transcription-polymerase chain reaction (PCR) test for SARS-CoV-2 (i.e., PCR-confirmed diagnosis) or the typical thoracic CT findings of COVID-19 as defined by the British Society of Chest Radiology (i.e., radiological diagnosis) (Figure 1) (6).
The Wells score and the pulmonary embolism severity index (PESI) were calculated and classified using the 2019 guidelines of the European Society of Cardiology. Accordingly, the patients were classified as having low, intermediate-low, intermediate-high, or high risk (7).
Statistical analysis
Categorical variables were presented as numbers and percentages and compared with the chi-square or Fischer’s exact test. Quantitative variables were given as mean ± standard deviation and median [interquartile range (IQR)] values. Coefficient of variation (<20%), kurtosis/standard error (<1.96), skewness/standard error (<1.96) ratios, visual (histogram and detrended Q-Q plot graphs) and analytical (Kolmogorov-Smirnov/Shapiro-Wilk tests) methods were utilized to evaluate whether the data were normally distributed. Comparisons between groups were undertaken using the independent-samples t-test (Student-t-test) for variables with a normal distribution, and the Mann-Whitney U test for those that were not normally distributed.
Multivariate logistic regression models were built to determine factors associated with transfer to the intensive care unit. The factors included in the model were age, gender, comorbidity count, presence of malignancy, Wells and PESI scores, localization and side of the embolus/emboli, d-dimer, troponin I, and CRP. The goodness-of-fit of the model was tested with the Hosmer-Lemeshow test. Laboratory tests with high missing data (ferritin, LDH, fibrinogen, procalcitonin, and interleukin-6) and variables that were highly correlated with d-dimer, troponin I, and CRP (lymphocyte count, NLR, and platelet count) were not included in the model even if they were significant in pairwise comparisons. Furthermore, oxygen support needs and related treatments were not included in the regression analysis due to the possibility of missing data before or after transfer to the intensive care unit. Lastly, blood pressure and pulse were excluded from the model since they were already included in the calculation of the PESI score.
The statistical significance level was taken as p < 0.05 (two-sided). No imputation was provided for the missing data. Statistical analyses were performed using the the Statistical Products and Service Solutions for the Social Sciences version 23 (IBM SPSS®, Armonk, New York, USA) software package.
Ethical consideration
Ethical approval for the study was obtained from the Clinical Research Ethics Committee of Ankara City Hospital (approval number: E1-21-1628, date: March 17, 2021). The study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was not obtained as the data were retrospectively obtained from electronic records without collecting patients’ identity information. The results of this study were presented as an oral presentation at the Ankara City Hospital 2021 Annual Congress (online congress) (the Turkish Respiratory Society 2021 Annual Congress (online congress) (October 29 to November 3, 2021) (SS-131).
Results
The study included a total of 123 patients diagnosed with COVID-19-related APE from April 2020 to April 2021, and analyses were performed on the data of all these patients. Eighty-three (66.9%) of the patients were male, and the mean age was 61.89 ± 16.09 years. No patient was diagnosed with APE before hospitalization. They were not previously receiving anticoagulants.
Thirty-eight (30.8%) patients were treated and followed up in the intensive care unit. Age, number of comorbidities, and pulse were found to be statistically higher in patients who were followed up in the intensive care unit (p = 0.002, p = 0.032, and p < 0.001, respectively). COVID-19 was diagnosed by PCR in 69.9% of patients and by typical thorax CT image in the rest. Table 1 presents the demographic and clinical data and distribution of vital signs at the time of admission for patients diagnosed with COVID-19-related APE according to their intensive care unit admission.
| APE: Acute pulmonary thromboembolism, ICU: Intensive care unit, OR: Odds ratio, CI: Confidence interval, IQR: Interquartile range, SD: Standard deviation, CT: Computed tomography; *Student’s t-test. | ||||
| Table 1. Demographic and clinical data and distribution of vital signs at admission in patients with COVID-19-related APE according to the ICU transfer status | ||||
|
|
|
|
|
|
| Male gender |
|
|
|
|
| Comorbidity |
|
|
|
|
| Hypertension |
|
|
|
|
| Coronary artery disease |
|
|
|
|
| Diabetes mellitus |
|
|
|
|
| Malignancy |
|
|
|
|
|
|
|
|
|
|
| Age (years) (mean ± SD) |
|
|
|
|
| Number of comorbidities |
|
|
|
|
| Time from CT scan to COVID-19 diagnosis (days) |
|
|
|
|
| Body temperature (°C) |
|
|
|
|
| Pulse (/min) |
|
|
|
|
| Systolic blood pressure (mmHg) |
|
|
|
|
| Diastolic blood pressure (mmHg) |
|
|
|
|
When the laboratory values of the patients included in the study were examined at the time of the diagnosis of APE, lymphocyte count, platelet count, and albumin levels were found to be lower among those followed up in the intensive care unit. NLR, serum LDH, ferritin, troponin I, d-dimer, CRP, procalcitonin, interleukin-6, and CRP-to-albumin ratio were statistically significantly higher in the intensive care group. The laboratory findings of the groups are shown in Table 2.
| APE: Acute pulmonary thromboembolism, ICU: Intensive care unit, SD: Standard deviation, eGFR: estimated glomerular filtration rate, CRP: C-reactive protein, pH: power of Hydrogen, pO2: partial oxygen pressure, pCO2: partial carbon dioxide pressure, HCO3: bicarbonate concentration, SO2: oxygen saturation; * Student’s t-test; Data presented as median (interquartile range) or mean ± standard deviation. | ||||
| Table 2. Distribution of laboratory data in patients with COVID-19-related APE according to the ICU transfer status | ||||
|
|
|
|
|
|
| Leucocyte (/µL) |
|
|
|
|
| Neutrophil leukocyte (/µL) |
|
|
|
|
| Lymphocyte leukocyte (/µL) |
|
|
|
|
| Neutrophil-to-lymphocyte ratio |
|
|
|
|
| Hemoglobin (g/dL) |
|
|
|
|
| Hematocrit (%) |
|
|
|
|
| Thrombocyte (x1,000/µL) |
|
|
|
|
| Serum creatinine (mg/dL) |
|
|
|
|
| eGFR (ml/min) |
|
|
|
|
| Serum lactate dehydrogenase (U/l) |
|
|
|
|
| Serum albumin (mg/L) |
|
|
|
|
| Ferritin (µg/L) |
|
|
|
|
| Troponin I (ng/L) |
|
|
|
|
| Brain natriuretic peptide (ng/mL) |
|
|
|
|
| Activated partial thromboplastin time (sec) |
|
|
|
|
| Prothrombin time (sec) |
|
|
|
|
| Fibrinogen (mg/dL) |
|
|
|
|
| D-dimer (ng/mL) |
|
|
|
|
| CRP (mg/L) |
|
|
|
|
| Procalcitonin (ng/mL) |
|
|
|
|
| Interleukin-6 (pg/mL) |
|
|
|
|
| CRP/Albumin ratio |
|
|
|
|
| pH |
|
|
|
|
| pO2 (mmHg) |
|
|
|
|
| pCO2 (mmHg) |
|
|
|
|
| HCO3 (mol/L) |
|
|
|
|
| sO2 (%) |
|
|
|
|
The rates of high Wells and PESI scores, PESI class 5, and high risk for early mortality were higher in the intensive care group. Table 3 presents the distribution of the diagnostic and prognostic scores and imaging findings according to transfer to the intensive care unit.
| APE: Acute pulmonary thromboembolism, ICU: Intensive care unit, OR: Odds ratio, CI: Confidence interval, IQR: Interquartile range, PESI: Pulmonary embolism severity index, SD: Standard deviation; *Student’s t-test. | ||||
| Table 3. Distribution of diagnostic and prognostic scores in patients with COVID-19 related APE according to the ICU transfer status | ||||
|
|
|
|
|
|
| Deep vein thrombosis |
|
|
|
|
| Median Wells score (IQR) |
|
|
|
|
| PESI score (mean ± SD) |
|
|
|
|
| PESI class |
|
|||
| Class I (reference) |
|
|
|
|
| Class II |
|
|
|
|
| Class III |
|
|
|
|
| Class IV |
|
|
|
|
| Class V |
|
|
|
|
| Early mortality risk assessment |
|
|||
| Low (reference) |
|
|
|
|
| Intermediate-low |
|
|
|
|
| Intermediate-high |
|
|
|
|
| High |
|
|
|
|
| Embolus localization |
|
|||
| Subsegmental artery (reference) |
|
|
|
|
| Segmental artery |
|
|
|
|
| Lobar artery |
|
|
|
|
| Main pulmonary artery |
|
|
|
|
| Laterality of embolism |
|
|||
| Unilateral (reference) |
|
|
|
|
| Bilateral |
|
|
|
|
| Pulmonary artery pressure (mmHg) (mean ± SD) |
|
|
|
|
| Median ejection fraction (%) (IQR) |
|
|
|
|
The oxygen need was higher among patients who were transferred to the intensive care unit compared to those not transferred (p < 0.001). It was also determined that the use of methylprednisolone (>250 mg/day) and colchicine were significantly higher in the intensive care group (p < 0.001 and p = 0.005, respectively). The median length of stay was 16.0 (IQR = 19) in patients who needed intensive care and 9.0 (IQR = 8) days in those without this need (p < 0.001). Mortality occurred in 12 (31.5%) patients followed up in the intensive care unit. The distribution of the treatments applied for COVID-19 and the clinical outcomes of the patients in both groups are shown in Table 4.
| APE: Acute pulmonary thromboembolism, ICU: Intensive care unit, OR: Odds ratio, CI: Confidence interval, HFNO: High-flow nasal oxygen, NIMV: Non-invasive mechanical ventilation, IMV: Invasive mechanical ventilation, IQR: Interquartile range. | ||||
| Table 4. Distribution of treatments applied for COVID-19 and clinical outcomes in patients with COVID-19-related APE according to the ICU transfer status | ||||
|
|
|
|
|
|
| Oxygen support |
|
|||
| None (reference) |
|
|
|
|
| Nasal cannula |
|
|
|
|
| Mask |
|
|
|
|
| Reservoir mask |
|
|
|
|
| HFNO |
|
|
|
|
| NIMV |
|
|
|
|
| IMV |
|
|
|
|
| Methylprednisolone (≥250 mg) |
|
|
|
|
| Colchicine |
|
|
|
|
| Hydroxychloroquine |
|
|
|
|
| Favipiravir | ||||
| Median length of stay (days) (IQR) (n = 123) |
|
|
|
|
| Mortality |
|
|
|
|
A multivariate logistic regression analysis was performed to identify factors affecting intensive care unit admission among hospitalized patients diagnosed with COVID-19-related APE. According to both univariate and multivariate analyses, the Wells score, the PESI score, and the CRP level statistically significantly increased the risk of intensive care needs. The detailed results are given in Table 5.
| APE: Acute pulmonary thromboembolism, OR: Odds ratio, CI: Confidence interval, adj.: adjusted, PESI: Pulmonary embolism severity index; *This analysis was performed on the data of 98 patients. Nagelkerke R2 = 0.63, Hosmer-Lemeshow test p-value = 0.81 | ||||||
| Table 5. Logistic regression analyses of factors affecting the risk of intensive care unit transfer in hospitalized patients with COVID-19-related APE | ||||||
|
|
|
|||||
|
|
|
|
|
|
|
|
| Age (years) |
|
|
|
|
|
|
| Male gender |
|
|
|
|
|
|
| Number of comorbidities |
|
|
|
|
|
|
| Malignancy |
|
|
|
|
|
|
| Wells score |
|
|
|
|
|
|
| PESI score |
|
|
|
|
|
|
| Embolus localization | ||||||
| Subsegmental (reference) |
|
|
||||
| Segmental |
|
|
|
|
|
|
| Lobar |
|
|
|
|
|
|
| Pulmonary artery |
|
|
|
|
|
|
| Embolism laterality (bilateral vs unilateral) |
|
|
|
|
|
|
| d-dimer |
|
|
|
|
|
|
| Troponin I |
|
|
|
|
|
|
| C-reactive protein |
|
|
|
|
|
|
Discussion
In this study, it was determined that high CRP levels and Wells and PESI scores in adult hospitalized patients with a diagnosis of COVID-19-related APE were associated with transfer to the intensive care unit. To the best of our knowledge, there is no study in the literature examining factors associated with intensive care needs among patients with a diagnosis of COVID-19-related APE. However, as another negative outcome showing parallelism to the intensive care needs, mortality has been previously investigated in patients with APE. In the absence of data on COVID-19-related APE, we referred to the available findings in the literature concerning the relationship between APE and adverse clinical outcomes to compare our results.
In a study in which the PESI scale was validated for the prediction of in-hospital mortality in patients diagnosed with COVID-19-related APE, 34.9% of the patients were followed up in the intensive care unit, and 17.6% died (8). In the current study, 30.8% of the patients were transferred to the intensive care unit, and 12.1% died. In this respect, the clinical outcomes obtained from our study are consistent with the literature and even indicate slightly lower intensive care needs and mortality rates.
The Wells score is used to predict the diagnosis of APE (9). In this study, it was determined that each point increase in the Wells score in patients diagnosed with COVID-19-related APE increased their probability of intensive care unit transfer by 1.7 times (OR = 1.70; 95% CI = 1.13 - 2.56; p = 0.011). In addition to the diagnostic use of the Wells score, its association with transfer to the intensive care unit in patients with COVID-19-related APE suggests that this parameter may also have a place in determining the prognosis of these patients. To the best of our knowledge, there is no study in the literature examining the utility of the Wells score in predicting the prognosis of patients diagnosed with COVID-19-related APE or their intensive care unit needs.
In this study, the mean and standard deviation values of the PESI score were found to be higher in patients diagnosed with COVID-19-related APE followed up in the intensive care unit compared to those without intensive care needs (120.34 ± 39.59 vs. 79.06 ± 27.56) (p < 0.001). Multivariate analysis revealed that each point increase in the PESI score increased the probability of intensive care unit transfer by 1.03 times (95% CI: 1.00–1.07; p = 0.048). In a multicenter retrospective cohort study conducted in Spain and France, factors determining in-hospital mortality were examined, and it was reported that a simplified PSI score of 1 or greater in patients with PCR-confirmed COVID-19 who presented with APE increased in-hospital mortality by 2.23 times (95% CI = 1.29 - 3.86) (10). Although the extent of the association may seem different due to the previous authors’ use of the simplified version of PESI, the direction, and significance of this association are similar. In another study conducted in Türkiye before COVID-19 to evaluate patients with APE, it was found that a high simplified PESI score increased long-term mortality (30 days and later) by 1.69 times (OR = 1.05; 95% CI = 0.98 - 1.12; p=0.165) (11), which supports the findings of the current study.
We found that the rate of embolism in the main pulmonary artery was significantly higher in patients who were transferred to the intensive care unit compared to those without an intensive care need. In the presence of variables such as oxygen support and respiratory rate in the PESI score, localization seems to lose its statistical significance in multivariate analysis. Similar to the findings of our study, a multicenter study conducted in Spain and France did not detect a statistically significant relationship between embolism localization and in-hospital mortality (10).
Arterial blood gas results of almost all patients who were transferred to the intensive care unit were available from the electronic recording system, whereas the results of about half of the patients who were not transferred to the intensive care unit were available. It is considered that the statistical significant difference between these groups could not be shown because most of the available arterial blood gas results were taken under oxygen therapy, the group in which arterial blood gas results were taken in the group that was not transferred to the intensive care unit probably had a more severe clinic, and the number of the group with arterial blood gas results was relatively low (type 2 error).
In a study that investigated the prognostic value of the serum albumin level in 269 patients with APE, it was shown that a low serum albumin level also increased long-term mortality (11). Consistently, in the current study, the serum albumin value was found to be lower in patients with COVID-19-related APE who were transferred to the intensive care unit compared to those who were not transferred to intensive care (p < 0.001).
In this study, the CRP level was higher in patients followed up in the intensive care unit compared to the remaining patients (p = 0.001). In a meta-analysis examining laboratory parameters with early prognostic value in COVID-19, elevated white blood cell count, CRP, lactate dehydrogenase, and d-dimer levels and a low lymphocyte count were found to increase mortality (12).
In our sample, the median d-dimer value was found to be 12.75 (IQR: 20.91) mg/L in patients followed up in the intensive care unit and 3.55 (6.65) mg/L in those who did not need intensive care, and the difference between the two groups was statistically significant (p < 0.001). When factors affecting intensive care needs were examined in a binary regression analysis, d-dimer was determined to be statistically significant in univariate analysis, while this significance was lost in multivariate analysis (OR: 1.05; 95% CI: 0.98-1.12; p = 0.165). Since d-dimer is a fibrin degradation product, it is associated with thrombus burden and prognosis (13,14). It also probably increases due to the inflammatory response and hypoxia-inducible transcription factor-dependent signaling pathway induced by SARS-CoV-2 infection (15). Therefore, we consider that d-dimer may have lost its significance in the presence of the PESI score and CRP, which represent the inflammatory response in our logistic regression model.
We determined that the NLR was higher in patients who were transferred to the intensive care unit compared to the other patient group. In a previous study, a high NLR was found to increase both 30-day and one-year mortality in patients with APE who were followed up in a center in Israel between 2007 and 2021 (16). Covering a period beginning before COVID-19 and spanning a portion of the pandemic, the study indicated that the NLR, which stands out as a factor in the prognosis of COVID-19 patients, is also essential in the prognostic assessment of APE.
When the CRP-to-albumin ratio was examined, the mean value was determined to be 23.71 ± 24.85 in patients followed up in the intensive care unit and 9.51 ± 17.02 in those who did not need intensive care. There was a statistically significant difference between the two groups (p < 0.001). In a previous study investigating the prognostic value of the CRP-to-albumin ratio in 186 patients diagnosed with APE in Türkiye, 54 patients were determined to be at moderate risk, 34 at high risk, and 98 at very high risk according to the PESI score. The PESI score had a moderate, positive correlation with the CRP-to-albumin ratio (r = 0.584, p < 0.0001) and troponin (r = 0.521, p < 0.0001, respectively) (17). Our findings are in agreement with the literature.
This study has certain limitations. First, data were collected retrospectively through the hospital’s computer system. Therefore, there were missing data due to some examinations not having been carried out within a pre-determined protocol. Nevertheless, the necessary data were obtained for many variables in most patients. Second, the single-center design of the study limits its external validity. Third, the small sample size limits the evaluation of rarer clinical outcomes, especially mortality. However, to the best of our knowledge, previous studies have only included a subgroup analysis of a very small number of patients for a special patient population, such as those with COVID-19-related APE. A strong aspect of the current study is that this special group exclusively constituted the sample, and the number of patients was higher than in previous subgroup analyses. Furthermore, we included patients diagnosed with APE by CTPA, which is considered the gold standard for the diagnosis of this condition. This minimized the possibility of a doubtful APE diagnosis. However, it is possible that some patients developed APE but did not undergo CTPA. Nevertheless, since the patients in this group are likely to have a milder clinical course, the association is biased toward the null.
In conclusion, this study showed that high Wells and PESI scores and serum CRP in hospitalized patients with COVID-19-related APE increased their probability of transfer to the intensive care unit. Therefore, it is recommended to monitor these patients more closely in terms of the development of intensive care needs.
Acknowledgments
We thank CTED Proof for the translation and proofreading of the manuscript.
Ethical approval
This study has been approved by the Ankara City Hospital No. 1 Clinical Research Ethics Committee (approval date: March 17, 2021, number: E1-21-1628). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. https://doi.org/10.1016/S0140-6736(20)30183-5
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-7. https://doi.org/10.1111/jth.14768
- Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298:E70-80. https://doi.org/10.1148/radiol.2020203557
- Republic of Türkiye Ministry of Health. COVID-19 (SARS-CoV-2 Enfeksiyonu) Antisitokin-Antiinflamatuar Tedaviler, Koagülopati Yönetimi. 2020. Available at: https://covid19.saglik.gov.tr/TR-66341/antisitokin-antiinflamatuar-tedaviler-koagulopati-yonetimi.html (Accessed on Apr 22, 2023).
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: jACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950-73. https://doi.org/10.1016/j.jacc.2020.04.031
- British Society of Thoracic Imaging. Thoracic imaging in COVID-19 infection. 2020. Available at: https://www.bsti.org.uk/media/resources/files/BSTI_COVID-19_Radiology_Guidance_version_2_16.03.20.pdf (Accessed on Apr 29, 2023).
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54:1901647. https://doi.org/10.1183/13993003.01647-2019
- Muñoz OM, Ruiz-Talero P, Hernández-Florez C, et al. Validation of the PESI scale to predict in-hospital mortality in patients with pulmonary Thromboembolism secondary to SARS CoV-2 infection. Clin Appl Thromb Hemost. 2022;28:10760296221102940. https://doi.org/10.1177/10760296221102940
- Kirsch B, Aziz M, Kumar S, et al. Wells score to predict pulmonary embolism in patients with coronavirus disease 2019. Am J Med. 2021;134:688-90. https://doi.org/10.1016/j.amjmed.2020.10.044
- Miró Ò, Jiménez S, Llorens P, et al. Pulmonary embolism severity and in-hospital mortality: an international comparative study between COVID-19 and non-COVID patients. Eur J Intern Med. 2022;98:69-76. https://doi.org/10.1016/j.ejim.2022.01.035
- Tanık VO, Çınar T, Karabağ Y, et al. The prognostic value of the serum albumin level for long-term prognosis in patients with acute pulmonary embolism. Clin Respir J. 2020;14:578-85. https://doi.org/10.1111/crj.13176
- Kiss S, Gede N, Hegyi P, et al. Early changes in laboratory parameters are predictors of mortality and ICU admission in patients with COVID-19: a systematic review and meta-analysis. Med Microbiol Immunol. 2021;210:33-47. https://doi.org/10.1007/s00430-020-00696-w
- Lerche M, Bailis N, Akritidou M, Meyer HJ, Surov A. Pulmonary vessel obstruction does not correlate with severity of pulmonary embolism. J Clin Med. 2019;8:584. https://doi.org/10.3390/jcm8050584
- Vahdat S. A review of pathophysiological mechanism, diagnosis, and treatment of thrombosis risk associated with COVID-19 infection. Int J Cardiol Heart Vasc. 2022;41:101068. https://doi.org/10.1016/j.ijcha.2022.101068
- Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77-83. https://doi.org/10.1016/j.thromres.2019.07.013
- Efros O, Beit Halevi T, Meisel E, et al. The prognostic role of neutrophil-to-lymphocyte ratio in patients hospitalized with acute pulmonary embolism. J Clin Med. 2021;10:4058. https://doi.org/10.3390/jcm10184058
- Özcan S, Dönmez E, Yavuz Tuğrul S, et al. The prognostic value of C-Reactive Protein/Albumin ratio in acute pulmonary embolism. Rev Invest Clin. 2022;74:097-103. https://doi.org/10.24875/RIC.21000547
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.






















