Abstract
Introduction: Predicting Air-Leak (AL) syndrome and knowing its risk factors in critically ill patients with Coronavirus Disease-2019 (COVID-19) can reduce morbidity and mortality. In this study, we aimed to investigate whether the thorax computed tomography severity score (CT-SS) can predict the development of AL.
Methods and Materials: COVID-19 patients were divided into two groups based on whether they developed AL syndrome. CT-SS was calculated for all patients. The predictive power of CT-SS for the development of AL syndrome and possible risk factors for AL were investigated.
Results: AL syndrome developed in 52 out of 272 patients included in the study (19.1%). The median CT-SS was significantly higher in the AL group. The CT-SS value in the AL group was 19 (2-25), while it was 13 (1-25) in the non-AL group (p-value <0.001). CT-SS was found to have good diagnostic performance in predicting the occurrence of AL (p<0.001). When comparing the ICU mortality rates of the groups, it was 42.3% in the non-AL group and 88.5% in the AL group.
Discussion and Conclusion: CT-SS may have predictive potential for the development of AL syndrome associated with COVID-19. However, further studies are needed in this area.
Keywords: COVID-19, intensive care unit, air-leak syndrome, CT-severity score, mortality
Introduction
The coronavirus disease caused by the COVID-19 virus has continued to be a global health threat in many countries since November 2020 (1). While most COVID-19 patients suffer from the disease with minor symptoms, some experience respiratory failure requiring invasive or non-invasive mechanical ventilation (2). These patients may experience severe life-threatening complications such as air-leak syndrome, including pneumothorax, pneumomediastinum, pneumopericardium, pneumoperitoneum, and subcutaneous emphysema (3). Air-leak syndrome (AL) is a clinical phenomenon associated with the leakage or escape of air from an air-containing space into areas that are usually air-free under normal conditions (4). Previous studies have documented elevated levels of hyperinflammation in patients with COVID-19 cases (5). Although the pathophysiology of air-leak syndrome is not clear, it is thought that air leaks resulting from alveolar rupture caused by direct alveolar injury facilitated by hyperinflammation may lead to broncho-vascular dissection (6). Also known as the Macklin effect, this results in the rupture of the alveolar tree associated with increased alveolar pressure and displacement of free air towards the hilum and mediastinum (6). Air advancing through the broncho-vascular sheath eventually diffuses into the pleural space, mediastinum, and subcutaneous tissue (7). Pneumothorax and pneumomediastinum can be seen in COVID-19 patients when barotrauma is avoided with lung protective ventilation, even in patients who are not mechanically ventilated (8). Even without traditional risk factors such as smoking and underlying lung disease, air leaks may occur in COVID-19 patients. The high mortality rate of AL syndrome, which is seen at a substantial rate, is clinically worrisome. To prevent the development of AL, diagnostic methods that can predict AL early are needed. With its high sensitivity rate, computed tomography is a beneficial method in imaging the pulmonary involvement of the disease (9). As our primary aim, we planned to investigate the predictive value of the Chest CT severity score (CT-SS), a semi-quantitative pulmonary involvement score based on computed tomography (CT) in the development of AL in mechanically ventilated COVID-19 patients in the ICU. Our second aim is to investigate the risk factors that play a role in the development of AL and the mortality rate of patients who develop AL.
Material and Methods
Study design and participants
As a retrospective analysis, after approval from the University Ethics Committee (Approval ID: 2022/38-10), this study consisted of 272 symptomatic patients in the tertiary intensive care unit for COVID-19 patients between 01.04.2020 and 01.09.2022. It was conducted on a cohort group and obtained by scanning electronic medical and laboratory data. Written informed consent was waived due to the nature of the study.
Inclusion criteria of the study
All patients admitted to the intensive care unit with positive COVID-19 polymerase chain reaction (PCR) tests, over 18 years old, who had chest CTs and developed AL, were included in the study.
Exclusion criteria of the study
Patients with negative PCR tests, under 18 years of age, pregnant or lactating, without chest CT scans, and those with isolated pneumothorax, pneumomediastinum, or subcutaneous emphysema were excluded from the study.
Data collection
The following data for each patient were scanned from the electronic hospital database: Data such as age, gender, body mass index, Charlson comorbidity index (CCI), Acute Physiology and Chronic Health Assessment (APACHE) II, and SOFA scores at ICU admission were collected. CT-SS, arterial blood gas analysis (arterial partial pressure of oxygen (PaO2); arterial partial pressure of carbon dioxide (PaCO2); FiO2; PaO2/FiO2 ratio; bicarbonate; laboratory data including hemogram parameters, C-reactive protein (CRP), procalcitonin, D-dimer, serum creatinine (sCr), total bilirubin, ferritin, hospital stay duration, length of stay in the ICU, and ICU and hospital mortality. In addition, to detect the inflammatory status of patients, The systemic immune-inflammatory index (SII) and neutrophil/ lymphocyte ratio (NLR) was calculated (5).
Chest computed tomography (CT) image acquisition and interpretation
A 64-channel multi-detector CT scanner (Brilliance, Philips Medical Systems) was used with the following imaging protocol: 120 kV, 80 mA, slice thickness 1 mm, and high spatial frequency reconstruction algorithm (bone algorithm), without intravenous contrast agent. All scans were reviewed for CT diagnosis of COVID-19-associated pneumonia. CT scans were classified according to the Expert Consensus Statement on Reporting of Chest CT Findings Related to COVID-19 of the North American Society of Radiology (RSNA) as follows: (1) negative for pneumonia, (2) typical appearance, (3) atypical appearance, and (4) indeterminate appearance (X) (10). A semi-quantitative scoring system was used to quantitatively predict the pulmonary involvement of CT scans that show a typical and uncertain outlook for COVID-19 (Y). Each of the five lung lobes was visually scored on a scale of 0 to 5, with 0 indicating no involvement; 1, less than 5% involvement; 2, 5–25% involvement; 3, 26%–49% involvement; 4, 50–75% involvement; and 5, more than 75% involvement. The total CT score was the sum of the individual lobar scores and ranged from 0 (no involvement) to 25 (maximum involvement) (11).
Statistical analysis
Statistical Package for the Social Sciences Version 24.0 (IBM Corporation, Armonk, NY, USA) was used to perform statistical analysis. The distribution of the groups was evaluated by Kolmogorov-Smirnov and Shapiro-Wilk tests. It was determined that the groups did not fit the normal distribution pattern. All continuous variables were expressed as median (minimum-maximum). Categorical variables were expressed as numbers (n) and percentages (%). Descriptive statistics were performed for all variables using the Kruskal-Wallis test, Mann-Whitney U test, χ2 test, or Fisher’s Exact test. Univariate and multivariate analyses were performed to evaluate the variables associated with the development of AL. The optimal cut-off point for HFNO day and CT score was sought to predict AL formation by analyzing diagnostic performance with receiver operating characteristic (ROC) curve analysis. A p-value of <0.05 was considered significant.
Results
Patients characteristics
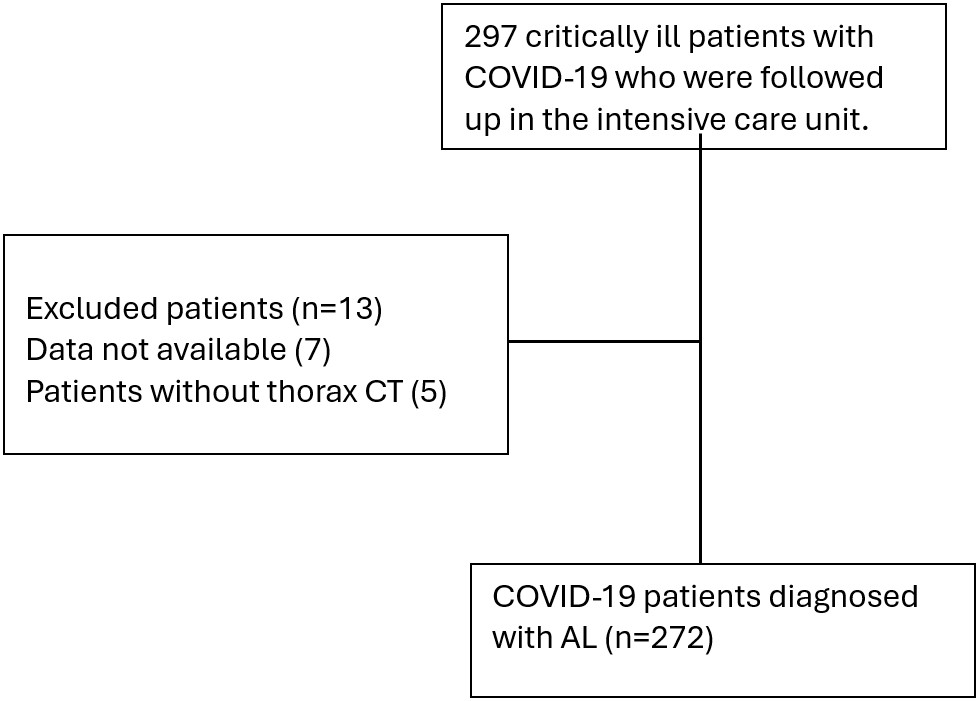
We evaluated 297 critically ill patients with COVID-19 who were followed up in the intensive care unit. Data from 272 patients who met the selection criteria for the study were evaluated (Figure 1). Air-leak syndrome occurred in 52 (19.1%) of these patients. Patients were divided into two groups according to whether AL developed or not. The median age was higher in the non-AL group compared to the AL group, 67 (34-92) and 71 (28-95) respectively (p=0.188). The median CT-SS was significantly higher in the AL group. The AL group’s Chest CT Severity Score value was 19 (2-25), and the non-AL group’s score was 13 (1-25) (p<0.001) (Table 1).
| All values were expressed as n(%) or median (IQR). BMI: Body mass index, APACHE II score: Acute Physiology and Chronic Health Assessment score, SOFA score: Sequential Organ Failure Assessment score, GCS: Glasgow coma scale, COPD: Chronic Obstructive Pulmonary Disease, ICU: Intensive Care Unit. *Calculated on the day of admission to ICU. | |||||
| Table 1. Demographic data, comorbidities, and clinical outcomes of the patients | |||||
|
|
|
|
|
||
| Age |
|
|
|
|
|
| Gender, male |
|
|
|
|
|
| BMI, kg/m2 |
|
|
|
|
|
| APACHE II score |
|
|
|
|
|
| SOFA score* |
|
|
|
|
|
| CCI |
|
|
|
|
|
| CT Severity score |
|
|
|
|
|
| Smokers | able |
|
|
|
|
| unable |
|
|
|
||
| ex-smoker |
|
|
|
||
| Comorbidities | |||||
| Hipertansion |
|
|
|
|
|
| Diabetes mellitus |
|
|
|
|
|
| COPD |
|
|
|
|
|
| Coronary artery disease |
|
|
|
|
|
| Chronic liver failure |
|
|
|
|
|
| Atrial fibrilation |
|
|
|
|
|
| Chronic renal failure |
|
|
|
|
|
| Cerebrovascular disease |
|
|
|
|
|
| Malignancy |
|
|
|
|
|
| Dementia |
|
|
|
|
|
| Parkinson's disease |
|
|
|
|
|
Laboratory data
For laboratory data, no significant difference was found between the groups, except for hemoglobin and lymphocyte values. Median hemoglobin values were 11.5 (6.7-17.8) mg/dl in the AL group, and 12.5 (5.6-16.9) in the non-AL group (p=0.009). Median lymphocyte values were 500 (100-1500) in the AL group, and 500 (0-5200) in the non-AL group (p=0.048). FiO2 and P/F ratio values were statistically significant at the time of first admission to the ICU. FiO2 values were 60 (30-90) mmHg in the AL group, and 60 (21-100) in the non-AL group (p=0.003). Median P/F ratio values were 106.5 (61-322) in the AL group, and 116 in the non-AL group (p=0.021) (Table 2).
|
All values were expressed as n (%) or median (IQR). WBC: Leukocyte; BUN, Blood Urea Nitrogen; CRP: C-Reactive Protein, BNP: Brain natriuretic peptide, SII:The Systemic immune-inflamatory index, NLR:Neutrophil/Lymphocyte ratio, PaCO2: Arterial partial pressure of carbon dioxide, PaO2: Arterial partial pressure of oxygen, FiO2: Presented oxygen ratio, P/F ratio: PaO2/ FiO2. *Values presented are when patients are admitted to the ICU. |
||||
| Table 2. Laboratory data of the patients on the day of admission to the intensive care unit | ||||
| Laboratory Values* |
|
|
|
|
| WBC, x 103/µL |
|
|
|
|
| Haemoglobin, g/dL |
|
|
|
|
| Lymphocyte x 103/µL |
|
|
|
|
| Platelet, x 103/µL |
|
|
|
|
| BUN, mg/dL |
|
|
|
|
| Creatinin, mg/dL |
|
|
|
|
| CRP, mg/L |
|
|
|
|
| Ferritin, ng/mL |
|
|
|
|
| D-Dimer, µg/mL |
|
|
|
|
| Procalcitonin, ng/mL |
|
|
|
|
| BNP |
|
|
|
|
| SIII |
|
|
|
|
| NLR |
|
|
|
|
| Arterial Blood Gas Analysis | ||||
| pH |
|
|
|
|
| PaCO2 |
|
|
|
|
| PaO2 |
|
|
|
|
| Lactat |
|
|
|
|
| FiO2 |
|
|
|
|
| P/F Ratio |
|
|
|
|
Treatments and outcomes
The number of patients who underwent HFNC was 61.5% and 85%, respectively (p<0.001). The number of days HFNC was applied was 1 (0-15) days in the AL group and 4 (0-20) days in the non-AL group (p<0.001). The number of days NIV was administered was 2 (0-21) days in the AL group and 3.5 (0-14) days in the non-AL group (p=0.048). It was determined that while all patients in the AL group received IMV (100%), only 60% of patients in the non-AL group received IMV treatment (p<0.001). The number of days IMV was applied was 11 (1-26) days in the AL group, while it was 9 (0-32) days in the non-AL group (p=0.015). When the intensive care mortality of the groups was compared, it was found to be 42.3% in the non-AL group and 88.5% in the AL group. When hospital mortality was analyzed, it was found to be 51.6% in the non-AL group and 90.4% in the AL group. ICU and hospital mortality were significantly higher in the AL group (p<0.001) (Table 3).
| All values are expressed as n (%) or median (IQR). HFNO: High Flow Nasal Oxygen, NIV: Non-invasive mechanical ventilation, IMV: Invasive mechanical ventilation, PC-SIMV:Pressure Controlled-Synchronized Intermittent Mandatory Ventilation, PC-APRV:Pressure Controlled-Airway Pressure Release Ventilation, VC-AC: Volume Control-Asist Control, VC-SIMV: Volume Control- Synchronized Intermittent Mandatory Ventilation, ECMO: Extracorporeal Membrane Oxygenation, VAP:Ventilator Associated Pneumonia, ARDS: Acute Respiratuar Dystress Syndrome, AKI: Acute Kidney Injury, ICU: Intensive Care Unit. | ||||
| Table 3. Significant events and treatment modalities | ||||
|
|
|
|
|
|
| Respiratory Support Time | ||||
| HFNC day |
|
|
|
|
| NIV day |
|
|
|
|
| IMV day |
|
|
|
|
| IMV able |
|
|
|
|
|
IMV mode names -PC-SIMV -PC-APRV -VC-AC -VC-SIMV -Others |
|
|
|
|
|
IMV mod type. -Volume control -Pressure control |
|
|
|
|
| ECMO |
|
|
|
|
| Vasopressor need |
|
|
|
|
| VAP |
|
|
|
|
| ARDS |
|
|
|
|
| Acute Cardiac Injury |
|
|
|
|
| Tracheostomy |
|
|
|
|
| Septicemia/septic shock |
|
|
|
|
| AKI |
|
|
|
|
| Number of patients admitted to the ICU as intubated |
|
|
|
|
| Number of patients who underwent NIV |
|
|
|
|
| Number of patients who underwent HFNC |
|
|
|
|
| Length of stay (days) | ||||
| Time to intubation in the ICU |
|
|
|
|
| Hospitalization time from the first symptom |
|
|
|
|
| Mortality |
|
|||
| ICU |
|
|
|
|
| Hospital |
|
|
|
|
Risk factors for air-leak syndrome
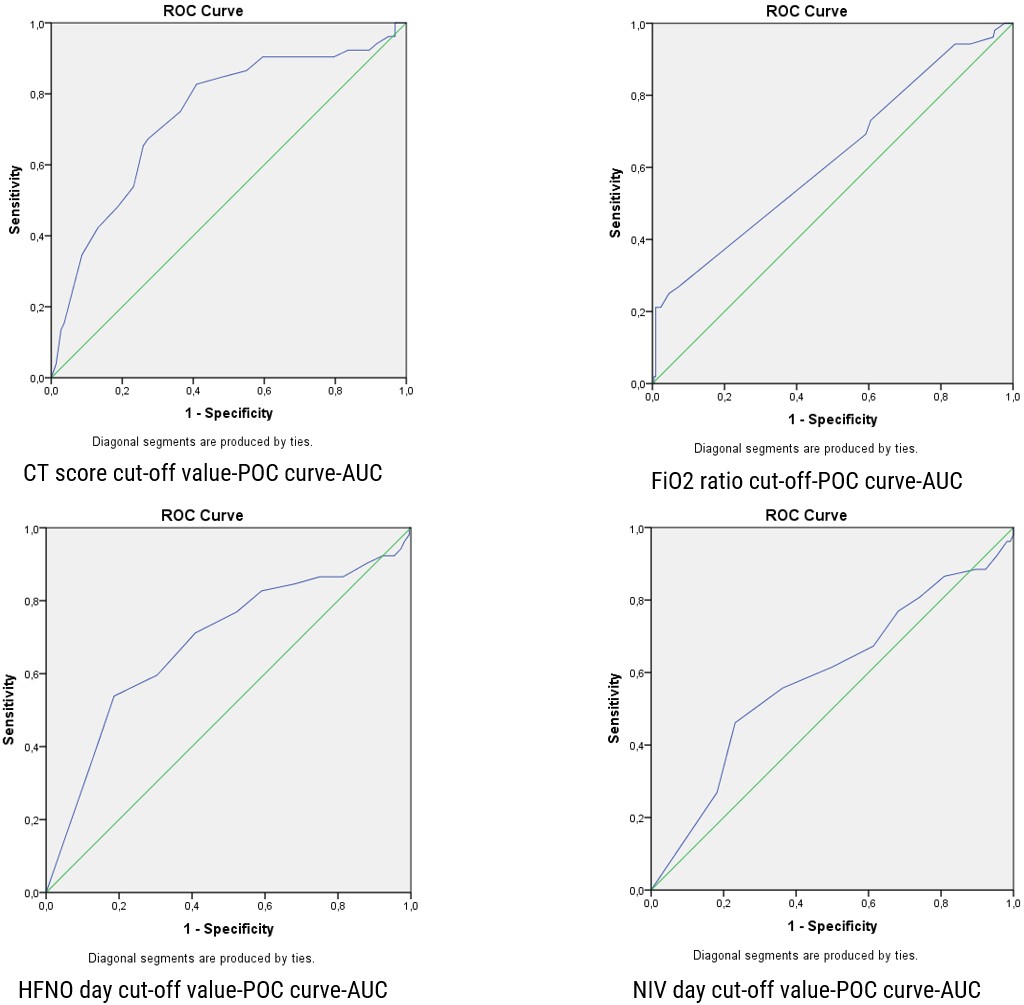
CT severity score and HFNC days were shown to have good diagnostic performance in predicting the occurrence of AL, with an area under the ROC of 0.739 for CT score and 0.684 for HFNC days, with 95% confidence intervals (CI) of 0.661-0.817 and 0.596-0.771, respectively (p<0.001). The optimal cut-off point for HFNC days was 3.5 and 15.5 for the CT severity score (Figure 2). Confounders included in the logistic regression analysis were as follows: FiO2 on the first hour of therapy, CT-SS, HFNC days, NIV days, and P/F ratio. Among these, FiO2 on the first hour of therapy 57.2 (0.625 [0.536-0.714], p=0.005), NIV days 2.5 (0.588 [0.497-0.679], p=0.049), and P/F ratio 112.5 (0.603 [0.511-0.695], p=0.021) with 95% confidence intervals (CI) were independent risk factors for the occurrence of AL (Table 4). The sensitivity and specificity of the CT severity score are 75% and 63.6%, respectively, and for HFNC days are 71.2% and 59.1%, respectively (Table 4). Neutrophil/lymphocyte ratio (NLR) were calculated (5).
| CI: Confidence interval | |||||
| Table 4. Analysis of independent risk factors for the development of AL in critically ill patients with COVID-19 | |||||
| Risk Factors |
|
|
|
|
|
| CT-SS |
|
|
|
|
|
| FiO2 |
|
|
|
|
|
| HFNC days |
|
|
|
|
|
| NIV days |
|
|
|
|
|
| P/F ratio |
|
|
|
|
|
Discussion
In this presented study, 272 COVID-19 patients were evaluated, and we found that 19.2% developed AL. The cases we define as AL syndrome in our study are cases in which all three clinical conditions (pneumothorax, pneumomediastinum, and subcutaneous emphysema) are observed together. We defined that chest CT severity score had good performance in predicting the development of AL. As a result of our analysis, we concluded that chest CT severity score predicted the development of AL with a cut-off value of 15.5. In addition, while the mortality rate in the intensive care unit was 42.3% in the group that did not develop AL, the mortality rate was 88.5% in the group that developed AL.
As our primary aim, we planned to investigate the predictive value of CT-SS, a semi-quantitative pulmonary involvement score based on computed tomography in the development of AL in mechanically ventilated COVID-19 patients in the ICU. Computed tomography (CT) is the gold standard in diagnosing AL and can distinguish bullous disease from pneumothorax (12). In contrast, chest X-ray, although inexpensive and rapid, has a pooled sensitivity of 52-60% and a specificity of 88-95% for diagnosing pneumothorax and pneumomediastinum (13,14). The high sensitivity of CT to diagnose AL in COVID-19 patients has led to the development of a semi-quantitative score that detects COVID-19 pulmonary involvement. Our study determined that the AL group’s median CT-SS was significantly higher. In addition, we found the cut-off value of CT-SS as 15.5, with a sensitivity of 75% and a specificity of 63.6% for the development of AL in patients followed up in the ICU for COVID-19. With this result, it can be thought that CT-SS has significant clinical value in detecting AL and may help diagnose patients who need more aggressive treatment. In a meta-analysis examining data from 7106 previous COVID-19 patients, the pooled estimate as the cut-off value for the optimal predictive value of CT-SS and mortality was calculated as 7,124 (95% CI 5,307–9,563) (15). In a study investigating 148 Iranian COVID-19 patients, the predictive CT-SS cut-off for mortality was calculated as 15.5 points, with a sensitivity of 51.6-70.8% and a specificity of 72.6-% (16). Therefore, in addition to the typical radiological signs for COVID-19 patients, CT-SS should be routinely included in radiological reports. If this score is greater than 15.5, it may predict that critical results such as AL development may occur. According to the authors’ literature knowledge, there is no study investigating the success of CT-SS in predicting the development of AL in COVID-19 patients.
Our second aim in this study was to investigate both the risk factors that play a role in the development of air-leak syndrome and the mortality rates of these patients. The pathogenesis of AL development in COVID-19 patients is unclear (14). In our intensive care unit, HFNO or NIV treatment was applied to patients who developed ARF but did not have an indication for intubation. None of the patients developed AL during HFNO administration. In the analysis of independent risk factors contributing to the development of air-leak syndrome, we determined that the cut-off value was less than 3.5 days of HFNO treatment. HFNO can be safely applied in selected COVID-19 patients. Consistent with our finding, patients who developed AL were treated with HFNO and IMV, non-severe ARDS cases were treated with HFNO, and 76% of patients recovered after a median follow-up of 5 days (17). Among severe ARDS cases, the cure rate of pneumomediastinum/pneumothorax was 70% with the HFNO approach and 10% with IMV. In our study, it was determined that AL developed on the median second day of treatment in 8 patients who were treated with NIV. In the independent risk factor analysis for the development of AL, we determined the cut-off value to be less than 2.5 days of NIV therapy. In our analysis, we identified short-term application of HFNO and NIV as an independent risk factor. We attributed this to the rapid deterioration of respiratory parameters in patients with poor respiratory conditions, leading to rapid transition to invasive mechanical ventilation. In clinical practice, these patients who developed AL experienced rapid deterioration in respiratory parameters, short-term non-invasive mechanical ventilation support, and prolonged invasive mechanical ventilation support.
Since the beginning of the pandemic, case reports and case series have indicated that AL (air leaks) can occur spontaneously or post-intubation during the course of the disease (12). The variety of cases suggests that the mechanism of occurrence is not only related to mechanical ventilation-induced barotrauma but also that COVID-19 itself may predispose patients to AL (18).
In COVID-19 syndrome, the absence of invasive mechanical ventilation in 30-40% of patients who develop AL has led to investigations of the responsible mechanisms (19). In a study conducted in London on COVID-19 patients investigating subcutaneous emphysema, pneumomediastinum, and pneumothorax, the responsible causes were explained by several mechanisms (20). In COVID-19, edema and atelectasis reduce lung volume (21). This condition can cause damage due to regional overdistension in the lung (volutrauma), increased shear stress in ventilated alveolar tissue (atelectrauma), high transpulmonary pressures (barotrauma), and damage from surfactant dysfunction and inflammation (biotrauma) (22). Each of these processes can contribute to the formation of subcutaneous emphysema, pneumomediastinum, and pneumothorax in critically ill COVID-19 patients. In a retrospective study, a high proportion of patients who developed AL were managed with non-invasive ventilation as the initial advanced respiratory support instead of invasive mechanical ventilation. In patients managed with NIV, neither tidal volume nor large swings in transpulmonary pressure resulting from spontaneous respiratory efforts can be limited (20). This situation can combine with the decreased functional lung volume seen in COVID-19, leading to patient-self inflicted lung injury (P-SILI) (23). In this study, it was determined that patients managed with NIV were exposed to significantly high tidal volumes before the development of AL, which over time, resulted in P-SILI (24).This occurred despite patients maintaining acceptable oxygen saturation levels and arterial partial pressures of oxygen (20). In other words, during NIV therapy in COVID-19 patients, increased inspiratory effort and high tidal volumes due to P-SILI can drive the patient towards developing AL. The possibility of self-induced lung injury should be considered in patients spontaneously breathing during NIV therapy (25), as worsening lung injury increases respiratory drive, leading to more substantial inspiratory effort, creating a vicious circle in P-SILI (26).
Additionally, in another study investigating risk factors for the development of AL in patients treated with NIV for COVID-19, high-pressure support was found (27). Patient-ventilator dyssynchrony, increased respiratory effort, excessive cough reflex, and poor management of hyperactive delirium may increase the risk of AL (23,28). Another study detected high transpulmonary pressure values. In these patients, alveolar rupture may have resulted from repeated vigorous inspiratory efforts with significant decreases in pleural pressure and increases in transpulmonary pressure (29). Alveolar and pleural pressures move in opposite directions with the activity of the ventilator and the patient, and transpulmonary pressure can quickly become too high, potentially causing AL.
In their case series, Kayhan et al.(18), found the incidences of pneumothorax, pneumomediastinum, and subcutaneous emphysema to be 4.51%, 3.87%, and 5.16%, respectively. Despite maintaining plateau pressures below 30 cmH2O, they attributed the primary mechanism responsible for the development of AL not to barotrauma but to sudden increases in intrathoracic pressure and perforation of the alveolar wall (30). They also reported that dry cough could lead to air leaks in infected tissue due to increased intrathoracic pressure (18,31)
Our study found that IMV was performed on all patients who developed air leaks, even though the onset was during NIV treatment. Median IMV treatment was 11 days higher in the AL group. We found that the AL group’s highest rate was the dual-mode PC-APRV mode. Again in the AL group, pressure-controlled modes for initiating inspiration were at a very high rate. We thought this situation might be related to the application of dual-mode or pressure-controlled modes in our clinic to patients who were evaluated as severe ARF due to low P/F ratio within the first hour of admission to the ICU in the AL group. The fact that the selection favors pressure-controlled modes may have led to bias. However, due to the nature of our retrospective study, we could not measure plateau pressure (Pplato), so we could not determine whether our patients were exposed to barotrauma. Knox et al.(32), in their study comparing the development of pneumothorax and pneumomediastinum in pre-pandemic and post-pandemic ARDS patients, found higher PEEP levels in COVID-19 patients (16 vs. 10 mmHg, p<0.001). Additionally, they reported that COVID-19 ARDS patients experienced similar rates of pneumothorax but more pneumomediastinum compared to pre-pandemic ARDS patients.
Similarly, in a study conducted in New York, pneumomediastinum and subcutaneous emphysema developed in approximately 1/5 of the patients when high tidal volumes and PEEP were applied to patients (33). In addition to mechanical ventilation modes, we believe focusing on tidal volume, respiratory rates, inspiratory flows, and mechanical power applied to the alveoli will be more efficient.
Limitation
Our study has limitations due to the nature of retrospective studies. In the study, pressure support, which can provide information about the inspiratory effort in non-invasive mechanical ventilation, and plateau pressure values that can indicate alveolar injury in invasive ventilation were not reported. In addition, applying dual and pressure-controlled modes in our clinic in COVID-19 cases with low P/F ratios and severe acute respiratory failure caused a bias against these modes.
Conclusion
As the number of COVID-19 patients increases, we will continue to encounter the clinical picture of AL. With its high mortality rate and affecting approximately 1/5 of COVID-19 patients followed in the ICU, this complication deserves further investigation. This condition, the pathophysiology of which is unclear, may be caused by lung injury induced by inflammation, P-SILI, non-invasive or invasive mechanical ventilation. We think that it should be kept in mind that AL may develop in cases with a CT-SS score higher than 15.5 for early diagnosis and prevention. However, we believe that further studies are needed on this subject.
Ethical approval
This study has been approved by the Dokuz Eylul University Non-Interventional Research Ethics Committee (approval date: 30.11.2022, number: 2022/38-10). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Shahsavarinia K, Rahvar G, Soleimanpour H, Saadati M, Vahedi L, Mahmoodpoor A. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in critically ill COVID-19 patients: A systematic review. Pak J Med Sci. 2022;38:730-5. https://doi.org/10.12669/pjms.38.3.5529
- Ochani R, Asad A, Yasmin F, et al. COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 2021;29:20-36.
- Quincho-Lopez A, Quincho-Lopez DL, Hurtado-Medina FD. Case Report: Pneumothorax and Pneumomediastinum as Uncommon Complications of COVID-19 Pneumonia-Literature Review. Am J Trop Med Hyg. 2020;103:1170-6. https://doi.org/10.4269/ajtmh.20-0815
- Adeyinka A, Pierre L. Air Leak. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Akboga SA, Gokce A, Hatipoglu M, et al. The relationship between mortality and inflammatory markers and the systemic immune inflammatory index in patients in the intensive care unit with a pneumothorax as a complication of COVID-19 disease. Ir J Med Sci. 2022;191:1931-6. https://doi.org/10.1007/s11845-021-02740-x
- Brito J, Gregório P, Mariani A, et al. Pneumomediastinum in COVID-19 disease: Outcomes and relation to the Macklin effect. Asian Cardiovasc Thorac Ann. 2021;29:541-8. https://doi.org/10.1177/02184923211010089
- Marsico S, Del Carpio Bellido LA, Zuccarino F. Spontaneous Pneumomediastinum and Macklin Effect in COVID-19 Patients. Arch Bronconeumol. 2021;57:67. https://doi.org/10.1016/j.arbres.2020.07.030
- Elhakim TS, Abdul HS, Pelaez Romero C, Rodriguez-Fuentes Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: a rare case and literature review. BMJ Case Rep. 2020;13:e239489. https://doi.org/10.1136/bcr-2020-239489
- Alsharif W, Qurashi A. Effectiveness of COVID-19 diagnosis and management tools: A review. Radiography (Lond). 2021;27:682-7. https://doi.org/10.1016/j.radi.2020.09.010
- Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020;35:219-27. https://doi.org/10.1097/RTI.0000000000000524
- Chang YC, Yu CJ, Chang SC, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236:1067-75. https://doi.org/10.1148/radiol.2363040958
- Juneja D, Kataria S, Singh O. Air leaks in COVID-19. World J Virol. 2022;11:176-85. https://doi.org/10.5501/wjv.v11.i4.176
- Ebrahimi A, Yousefifard M, Mohammad Kazemi H, et al. Diagnostic Accuracy of Chest Ultrasonography versus Chest Radiography for Identification of Pneumothorax: A Systematic Review and Meta-Analysis. Tanaffos. 2014;13:29-40.
- Rollas K, Çalışkan T, Yavuz T, Güldoğan IK. Pneumothorax and Pneumomediastinum in Patients admitted to Intensive Care Unit with COVID-19 Pneumonia: A Single-Center Retrospective Analysis. Journal of Cardio-Vascular-Thoracic Anaesthesia and Intensive Care Society. 2022;28:178-83. https://doi.org/10.14744/GKDAD.2022.54366
- Zakariaee SS, Salmanipour H, Naderi N, Kazemi-Arpanahi H, Shanbehzadeh M. Association of chest CT severity score with mortality of COVID-19 patients: a systematic review and meta-analysis. Clin Transl Imaging. 2022;10:663-76. https://doi.org/10.1007/s40336-022-00512-w
- Aziz-Ahari A, Keyhanian M, Mamishi S, et al. Chest CT severity score: assessment of COVID‑19 severity and short-term prognosis in hospitalized Iranian patients. Wien Med Wochenschr. 2022;172:77-83. https://doi.org/10.1007/s10354-022-00914-5
- Simioli F, Annunziata A, Polistina GE, Coppola A, Di Spirito V, Fiorentino G. The Role of High Flow Nasal Cannula in COVID-19 Associated Pneumomediastinum and Pneumothorax. Healthcare (Basel). 2021;9:620. https://doi.org/10.3390/healthcare9060620
- Kayhan O, Demirkıran O, Ürkmez S, Dikmen Y. Air leaks in COVID-19 pneumonia. Turk Gogus Kalp Damar Cerrahisi Derg. 2022;30:281-5. https://doi.org/10.5606/tgkdc.dergisi.2022.20763
- Nasa P, Juneja D, Jain R. Air leak with COVID-19 - A meta-summary. Asian Cardiovasc Thorac Ann. 2022;30:237-44. https://doi.org/10.1177/02184923211031134
- Jones E, Gould A, Pillay TD, et al. Subcutaneous Emphysema, Pneumomediastinum, and Pneumothorax in Critically Ill Patients With Coronavirus Disease 2019: A Retrospective Cohort Study. Crit Care Explor. 2020;2:e0210. https://doi.org/10.1097/CCE.0000000000000210
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-2. https://doi.org/10.1016/S2213-2600(20)30076-X
- Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am J Roentgenol. 2020;215:87-93. https://doi.org/10.2214/AJR.20.23034
- Esnault P, Cardinale M, Hraiech S, et al. High Respiratory Drive and Excessive Respiratory Efforts Predict Relapse of Respiratory Failure in Critically Ill Patients with COVID-19. Am J Respir Crit Care Med. 2020;202:1173-8. https://doi.org/10.1164/rccm.202005-1582LE
- Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323:2329-30. https://doi.org/10.1001/jama.2020.6825
- Battaglini D, Robba C, Ball L, et al. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. Br J Anaesth. 2021;127:353-64. https://doi.org/10.1016/j.bja.2021.05.024
- Pinto EF, Santos RS, Antunes MA, et al. Static and Dynamic Transpulmonary Driving Pressures Affect Lung and Diaphragm Injury during Pressure-controlled versus Pressure-support Ventilation in Experimental Mild Lung Injury in Rats. Anesthesiology. 2020;132:307-20. https://doi.org/10.1097/ALN.0000000000003060
- Tonelli R, Bruzzi G, Manicardi L, et al. Risk Factors for Pulmonary Air Leak and Clinical Prognosis in Patients With COVID-19 Related Acute Respiratory Failure: A Retrospective Matched Control Study. Front Med (Lausanne). 2022;9:848639. https://doi.org/10.3389/fmed.2022.848639
- Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9:239-50. https://doi.org/10.1016/S2213-2600(20)30552-X
- Boussarsar M, Protti A. Pulmonary air leak in COVID-19: time to learn from our mistakes. Intensive Care Med. 2022;48:1614-6. https://doi.org/10.1007/s00134-022-06866-z
- Zantah M, Dominguez Castillo E, Townsend R, Dikengil F, Criner GJ. Pneumothorax in COVID-19 disease- incidence and clinical characteristics. Respir Res. 2020;21:236. https://doi.org/10.1186/s12931-020-01504-y
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-81. https://doi.org/10.1016/S2213-2600(20)30079-5
- Knox DB, Brunhoeber A, Peltan ID, Brown SM, Lanspa MJ. Comparison of radiographic pneumothorax and pneumomediastinum in COVID-19 vs. non-COVID-19 acute respiratory distress syndrome. Intensive Care Med. 2022;48:1648-51. https://doi.org/10.1007/s00134-022-06816-9
- Housman B, Jacobi A, Carollo A, et al. COVID-19 ventilator barotrauma management: less is more. Ann Transl Med. 2020;8:1575. https://doi.org/10.21037/atm-20-3907
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.