Abstract
Objective
This study aimed to investigate the frequency of potential drug-drug interactions (pDDI) and the effect of the number of drugs used on pDDIs using the UpToDate drug interactions application.
Materials and Methods
Patients aged >12 years who were treated in the intensive care unit for 3 days in 2016 were included in the study. pDDIs were detected by entering the drugs used for >24 hours into the UpToDate application. The total number of mild, moderate, and severe pDDIs, number of medications used, length of stay, age, number of chronic diseases, mechanical ventilation (MV) support, hospitalization diagnoses, and APACHE II score were statistically compared.
Results
Although the pDDI was found to increase with the number of medications administered, it did not show an exact association with the number of days of hospitalization. However, it was higher among patients who received MV support, had a high APACHE II score, and died. pDDI was observed least in the postoperative follow-up group.
Conclusion
The pDDI increased as the number of medications used by critically ill patients increased.
Keywords: Critically ill patient, intensive care unit, drug-drug interaction, adverse drug reactions, UpToDate
Introduction
Drug-drug interactions are often unpredictable and undesirable, regardless of their positive or negative effects. Decreased absorption, decreased metabolism, kidney problems, and polypharmacy are among the reasons that increase drug-drug interactions in critically ill patients (1).
Many different types of medications are used in intensive care patients because of systemic diseases and organ failures (2). Drug-related adverse events are twice as frequent as in normal care (3). It has been reported that 23% of clinically important adverse events in intensive care unit (ICU) are related to drug-drug interactions (4). An excessive number of drugs increases the possibility of interaction (5, 6). As a result, morbidity and mortality increase (7).
Potential drug-drug interaction (pDDI) is the possibility of drugs changing each other’s effects, and it is possible to detect it with computer programs. 40-80% of patients are exposed to at least one pDDI during their stay in the ICU (8). The number of pDDIs is reportedly related to the number of medications taken daily (1).
pDDIs can be detected using programs such as Stockley’s Drug Interactions, Micromedex Drug Interactions, and Epocrates (1). In addition, mobile applications such as UpToDate (Lexicomp Drug Interactions) and MedScape, which can be accessed via smartphones and computers, are used to detect pDDIs (9, 10).
This study aimed to investigate the frequency of pDDI detected using the UpToDate Drug Interactions mobile application, the effect of the number of drugs used on pDDI, and its relationship with factors affecting intensive care mortality.
Materials And Methods
Design of the Study
Approval for the research was received from the Ethics Committee of Cerrahpaşa Faculty of Medicine (number: 419987, date: 08.11.2017). The study was planned as a retrospective cross-sectional study.
The study was performed in a single center in the 12-bed tertiary ICU of a university hospital. Patients admitted to intensive care in 2016 were included in the study. The treatment plans were scanned, and the names of the drugs used for each patient were recorded one by one in the Excel file.
The criteria for inclusion in the study were admission to intensive care, age >12 years, and treatment for 3 days or more. Patients whose files could not be accessed or whose treatment plans were missing were excluded from the study.
Patient length of stay, age, number of chronic diseases, mechanical ventilation (MV) support, hospitalization diagnosis, outcome, names and number of medications used daily, and acute physiology and chronic health evaluation II (APACHE II) scores were recorded. Drugs used for >24 hours were considered data. Medications prescribed in single doses were not recorded. During the analysis phase, hospitalization diagnoses were grouped under certain diagnostic groups.
All medications were obtained from the paper treatment plans. The names, routes of administration, and doses of the drugs were recorded in an Excel file, with a separate column for each patient each day. The treatment plan applied for each day was entered into the UpToDate mobile application, and the pDDIs that occurred on the relevant day were recorded. The same procedure was performed repeatedly for each patient on each hospitalization day, and pDDIs were recorded.
pDDI detection using UpToDate
The generic names or active ingredients and routes of administration of the drugs administered to a patient within a day were entered into the UpToDate (Lexicomp Drug Interactions) mobile application. The results were obtained for each pair of drugs with potential interactions grouped as A, B, C, D, and X. Group A was not included in the data. B-C interactions were recorded as mild, D interactions as moderate, and X interactions as severe. This process was repeated for each patient’s treatment plan each day. Interaction types and numbers were recorded on separate days.
The UpToDate screening tool uses different databases to detect the presence or absence of significant interactions for a given drug pair. If conflicting evidence is presented between these databases, scientific literature and prospectus information are used to provide clinical practice recommendations to clinicians (10). In the program, the pDDI is grouped as A, B, C, D, and X, and the interaction group of each drug pair is taken as the result output.
Meanings of groups A, B, C, D, and X:
In group A, there were no known drug-drug interactions.
In group B, the mentioned drugs may interact, but there is no clinical evidence of their concomitant use.
In group C, the indicated agents may interact with each other in a clinically significant manner. The benefits of using these two drugs together usually outweigh the risks. An appropriate monitoring plan should be established to prevent possible adverse effects. Dosage adjustments may be necessary for some patient groups.
In group D, the data suggest that the two drugs interact with each other in a clinically meaningful manner. Patient-specific evaluation should be conducted to determine whether the benefits of concomitant treatment outweigh the risks. Precautions should be taken to determine the benefits and/or minimal toxicity of the use of active substances. These actions include close monitoring, empirical dosage changes, and the selection of alternative agents.
In group X, the indicated agents may interact with each other in a clinically significant manner. The risks associated with the combined use of these agents generally outweigh the benefits. These agents are generally contraindicated.
Statistical Analysis
Each patient’s total mild, moderate, and severe pDDIs, length of stay, age, number of chronic diseases, MV support, hospitalization diagnosis groups, and total and average number of drug use were statistically compared with the APACHE II score. Patients are listed in order from least to most according to the total and daily number of medications used during hospitalization. The results were then divided into 5 consecutive groups. The mean of each group was calculated. The groups were statistically compared according to mild, moderate, and severe pDDI.
The suitability of the variables to normal distribution was examined using visual (histogram and probability graphs) and analytical methods (Kolmogorov-Smirnov Test). It was determined that not all data had a normal distribution. Descriptive analyses are presented as percentages, and mean ± standard deviation (SD) and median (minimum-maximum) values are given for continuous variables. In data that did not conform to the normal distribution, Mann-Whitney U test was used for comparison analyzes between two groups. Comparisons between more than two groups were performed using the Kruskal-Wallis test. Pairwise comparisons of groups with significant Kruskal-Wallis test results were performed using the Mann-Whitney U test with Bonferroni correction. The Friedman test was used to evaluate more than two repeated measurements in dependent groups. The results are within the 95% confidence interval, and the margin of statistical error is accepted as 0.05. Statistical evaluation was performed using the Statistical Package for Social Sciences (SPSS) for Windows 25.0 (IBM SPSS Inc., Chicago, IL) program.
Results
Patient Characteristics
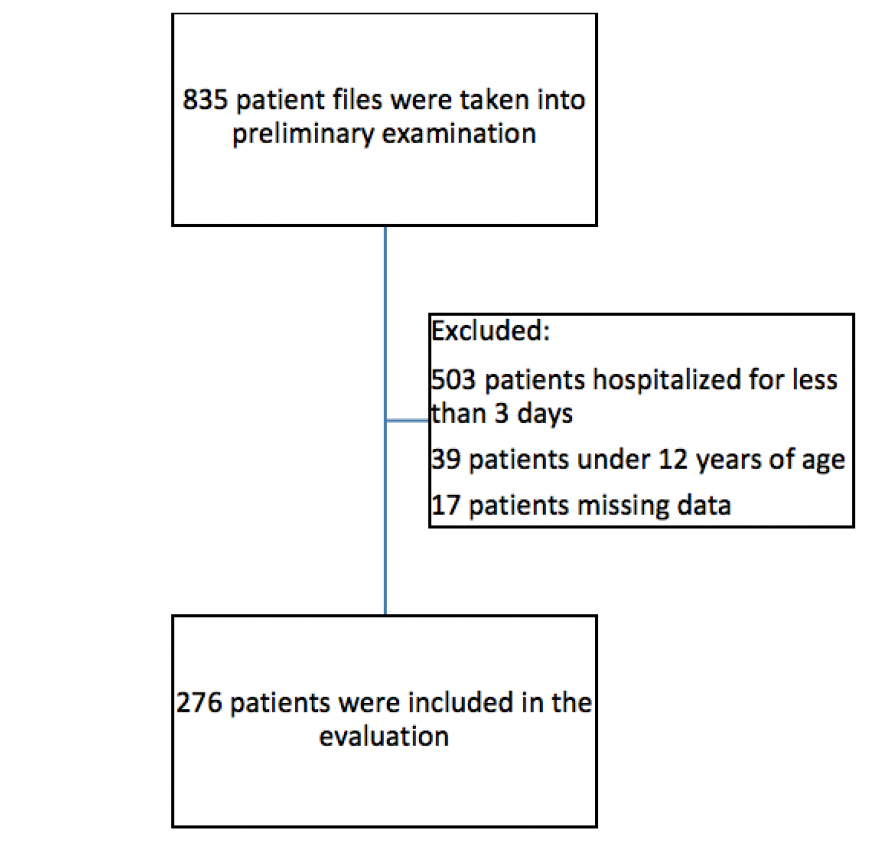
The number of patients admitted for treatment in the intensive care unit was 835. 276 of these patients were found suitable for statistical evaluation (Figure 1).
55.4% of the patients included in the study were male and 44.6% were female. The average age is 60.98±17.11 (age range 14-88). 62% of the patients were 60 years or older (Table 1).
| Table 1. Patient characteristics | |
|
- |
n(%) |
|
Male Age (Mean ± SD) BMI (Mean ± SD) |
153 (55.4) 60.98±17.11 24.60±5.89 |
|
Disease history |
|
|
Cardiovascular system Respiratory system Gastrointestinal system Neurological system Renal system |
54.7% 37% 31.2% 22.8% 19.9% |
|
Hospitalization diagnostic group |
|
|
Respiratory system disease Shock Postoperative follow-up Neurological system disease Urinary system disease |
118 (42.8) 89 (32.2) 35 (12.7) 25 (9.1) 9 (3.3) |
|
MV support |
|
|
There is None |
183 (66.3) 93 (33.7) |
|
Intensive care result |
|
|
Transfer to service Ex |
180 (65.2) 96 (34.8) |
|
Total length of stay |
|
|
Ort ±SS Ortanca (IQR) |
10.76±11.63 7 (5-12) |
|
Length of stay |
|
|
3-10 11-20 21-30 31 and over |
192 (69.6) 50 (18.1) 21 (7.6) 13 (4.7) |
|
Total number of ordered medicines |
|
|
Mean ± SD Median (IQR) |
111.61±130.85 69.00 (38.25-130.75) |
|
Daily number of ordered medicines |
|
|
Mean ± SD Median (IQR) |
9.77±3.18 9.59 (7.67-12.00) |
| SD: standard deviation, IQR: inter quartile range, MV: mechanical ventilation | |
The average APACHE II score was 22.22±7.35. Patients were divided into 5 groups according to hospitalization diagnosis (Appendix Tables 1, 2, 3).
The total mild pDDI was 28.5, and the daily mild pDDI was 3.32. The moderate pDDI was 3 and 0.36, respectively, while the severe interaction was zero for both (Table 2).
| Table 2. Potential drug-drug interaction-median (IQR) | ||
|
- |
Total |
Daily |
|
Mild interaction |
28.50(7.00-69.00) |
3.32(1.23-6.62) |
|
Moderate interaction |
3(0-9.00) |
0.36(0-1.00) |
|
Severe interaction |
0(0-0) |
0(0-0) |
| IQR: inter quartile range | ||
Total pDDI according to the total number of drugs
Patients were divided into 5 groups of 20% each according to the total number of medications ordered in the ICU (Appendix 1). Mild pDDI increased as the number of drug uses increased (p<0.001). In the pairwise group comparisons, except for the first-second and second-third group comparisons, the moderate pDDI increased as the number of drugs used increased (p<0.001). Severe pDDI was found to be higher in the group taking the most medication than in the two groups taking the least amount of medication (p<0.001). The pDDI decreased in all groups from mild to severe (Table 3).
| Table 3. Total pDDI according to total number of, median (IQR) | ||||
|
Total number of drugs Mean (SD) |
Mild |
Moderate |
Severe |
p-value2 |
|
1) 22.75±5.91 2) 44.69±7.91 3) 71.09±8.97 4)113.15±18.22 5) 302.91±183.94 |
3 (0-6) 9 (5-19.5) 32 (18.5-49) 53 (34.5-74.5) 138 (67.5-189.5) |
0 (0-1) 0 (0-4) 3 (0-7.5) 6 (1-10.5) 19.5 (6.5-35) |
0 (0-0) 0 (0-0) 0 (0-2.5) 0 (0-2) 0 (0-9) |
<0.001 <0.001 <0.001 <0.001 <0.001 |
|
p-value1 |
<0.001 |
<0.001 |
<0.001 |
- |
| 1: Tests used in comparison between groups: Kruskal-Wallis test, p<0.05 was considered significant. In pairwise group comparisons, Bonferroni correction was applied, and the Mann-Whitney U test was performed,2: Tests used for intra-group comparison: Friedman test, p<0.05 was considered significant.pDDI: potential drug-drug interaction, IQR: inter quartile range, SD: standard deviation | ||||
Daily pDDI according to daily drug number
Patients were divided into 5 groups of 20% each according to the daily number of medications ordered in the ICU (Appendix 1). It was determined that mild pDDI was lowest in the first group and second group, and there was no significant difference between the next three groups. The moderate pDDI was lowest in the first group, and the difference between the first and fifth groups was significant (p<0.001). The severity of pDDI was higher in the fifth group than in the first and second groups (p=0.001). The pDDI decreased from mild to severe in all groups (Table 4).
| Table 4. Daily pDDI according to the mean number of medications per day, median (IQR) | ||||
|
Mean number of drugs |
Mild |
Moderate |
Severe |
p-value2 |
|
1) 5.51±1.19 2) 8.01±0.48 3) 9.53±0.44 4) 11.34±0.67 5) 14.37±1.75 |
1 (0-1.83) 2.25 (0.82-3.66) 4.57 (2.53-7.06) 4.87 (3.20-9.54) 5.93 (3.20-8.40) |
0 (0-0.33) 0 (0-0.78) 0.50 (0-1.04) 0.75 (0.10-1.11) 0.90 (0.21-1.44) |
0 (0-0) 0 (0-0) 0 (0-0.42) 0 (0-0.23) 0 (0-0.49) |
<0.001 <0.001 <0.001 <0.001 <0.001 |
|
p-value1 |
<0.001 |
<0.001 |
0.001 |
- |
| 1: Tests used in comparison between groups: Kruskal-Wallis test, p<0.05 was considered significant. In pairwise group comparisons, Bonferroni correction was applied, and the Mann-Whitney U test was performed,2: Tests used for intra-group comparison: Friedman test, p<0.05 was considered significant.pDDI: potential drug-drug interaction, IQR: interquartile range | ||||
Total pDDI according to the length of stay
Patients were divided into 4 groups according to length of stay (Table 5). Moderate pDDI was found in less patients hospitalized for 3-10 days (p<0.001). Severe pDDI was more common in patients hospitalized for 21-30 days than in those hospitalized for 3-10 days (p=0.010). pDDI decreased from mild to severe interaction in all groups (p<0.001).
| Table 5. Total pDDI according to length of stay median (IQR) | ||||
|
Length of stay (days) |
Mild |
Moderate |
Severe |
p-value2 |
|
3-10 (n=192) 11-20 (n=50) 21-30 (n=21) 31 (n=13) |
14.00 (4-36) 62.50 (29-107) 143.00 (54-171) 279.00 (147-370) |
1.00 (0-5) 8.00 (2-19) 20.00 (7-34) 32.00 (11-61) |
0 (0-0) 0 (0-3) 0 (0-11) 0 (0-8) |
<0.001 <0.001 <0.001 <0.001 |
|
p-value1 |
<0.001 |
<0.001 |
0.010 |
- |
| 1: Tests used in comparison between groups: Kruskal-Wallis test, p<0.05 was considered significant. In pairwise group comparisons, Bonferroni correction was applied, and the Mann-Whitney U test was performed,2: Tests used for intra-group comparison: Friedman test, p<0.05 was considered significant.pDDI: potential drug-drug interaction IQR: interquartile range | ||||
Total pDDI according to the hospitalization diagnostic groups
Patients divided into 5 groups according to hospitalization diagnosis (Appendix 1) and were compared according to the degree of pDDI (Table 6). Mild and moderate pDDIs were observed less frequently in the postoperative follow-up group than in the neurological, respiratory, and shock groups (p<0.001). Severe pDDI was also less common in the postoperative follow-up group than in the respiratory system and shock groups (p=0.017). The pDDI decreased from mild to severe in all groups. Total pDDI results according to survival and MV are shown in Table 7.
| Table 6. Total pDDI according to hospitalization diagnosis median (IQR) | ||||
|
Hospitalization diagnosis group |
Mild |
Moderate |
Severe |
p-value2 |
|
Neurological system (n=25) Postoperative follow-up (n=35) Respiratory system (n=118) Shock (n=89) Urinary system (n=9) |
41 (26-73) 7 (3-19.5) 36 (9-76) 27 (7-73) 11 (7-36) |
8 (2-16) 0 (0-2) 3 (0-9) 3 (0-9) 3 (1-4) |
0 (0-0) 0 (0-0) 0( 0-2) 0 (0-1) 0 (0-0) |
<0.001 <0.001 <0.001 <0.001 0.001 |
|
p-value1 |
<0.001 |
<0.001 |
0.017 |
- |
| 1: Tests used in comparison between groups: Kruskal-Wallis test, p<0.05 was considered significant. In pairwise group comparisons, Bonferroni correction was applied, and the Mann-Whitney U test was performed,2: Tests used for intra-group comparison: Friedman test, p<0.05 was considered significant.pDDI: potential drug-drug interaction IQR: interquartile range | ||||
| Table 7. Total pDDI according to survival and mechanical ventilation median (IQR) | ||||
|
ICU Result |
Mild |
Moderate |
Severe |
p-value2 |
|
Alive (n=180) Exitus (n=96) |
16 (5-42) 57.5 (29.5-107) |
1.5 (0-7.5) 4.5 (2-15.5) |
0 (0-0) 0 (0-2) |
<0.001 <0.001 |
|
p-value1 |
<0.001 |
<0.001 |
0.107 |
- |
|
MV Support |
- |
|||
|
Yes (n=183) No (n=93) |
37 (12.5-84) 9 (4-36) |
4 (1-12.5) 0 (0-4) |
0 (0-1) 0 (0-0) |
<0.001 <0.001 |
|
p-value1 |
<0.001 |
<0.001 |
0.243 |
- |
| 1: Tests used in comparison between groups: Kruskal-Wallis test, p<0.05 was considered significant,2: Tests used for intra-group comparison: Friedman Test, p<0.05 was considered significant.pDDI: potential drug-drug interaction IQR: interquartile range, ICU: Intensive Care Unit, MV: Mechanical Ventilation | ||||
Total pDDI according to the number of systemic diseases
Patients were divided into 3 groups according to the number of systemic diseases they had before admission. Mild pDDI was found to be more common in groups with more systemic disease (p<0.001). There was no significant difference between the groups in terms of moderate pDDI (p=0.285). Severe pDDI was more common in patients with 3 or more systemic diseases than in those with no systemic disease (p=0.022). The pDDI decreased in all groups from mild to severe (Table 8).
| Table 8. Total pDDI according to the number of systemic diseases median (IQR) | ||||
|
Number of Systemic Diseases |
Mild |
Moderate |
Severe |
p-value2 |
|
No (n=23) 1-2 (n=138) ≥3 (n=115) |
6 (1.5-25.5) 24.5 (5-57) 39 (14.5-92) |
2 (0-9) 3 (0-8) 3 (0-12) |
0 (0-0) 0 (0-0) 0 (0-2.5) |
<0.001 <0.001 <0.001 |
|
p-value1 |
<0.001 |
0.285 |
0.022 |
- |
| 1: Tests used in comparison between groups: Kruskal-Wallis test, p<0.05 was considered significant. In pairwise group comparisons, Bonferroni correction was applied, and the Mann-Whitney U test was performed,2: Tests used for intra-group comparison: Friedman test, p<0.05 was considered significant.pDDI: Potential Drug-Drug Interaction, IQR: interquartile range | ||||
Total pDDI according to the APACHE II score
Patients were divided into 3 groups in terms of APACHE II score, and pDDI values were compared. Mild and moderate pDDI scores were higher in the 2 groups with an APACHE II score of 20 or more (p<0.001 and p<0.006). There was no significant difference in severe pDDI (p=0.713). The pDDI decreased in all groups from mild to severe (Table 9).
| Table 9. Total pDDI according to the APACHE II Score as a median (IQR) | ||||
|
APACHE II Score |
Mild |
Moderate |
Severe |
p-value2 |
|
0-19 (n=101) 20-29 (n=136) Thirty or more (n=39) |
14 (4-41) 30 (9-73) 45 (29.5-108.5) |
2 (0-6) 3 (0-10) 5 (1-18) |
0 (0-0) 0 (0-0) 0 (0-2) |
<0.001 <0.001 <0.001 |
|
p-value1 |
<0.001 |
<0.006 |
0.713 |
- |
| 1: Tests used in comparison between groups: Kruskal-Wallis test, p<0.05 was considered significant. In pairwise group comparisons, Bonferroni correction was applied, and the Mann-Whitney U test was performed,2: Tests used for intra-group comparison: Friedman test, p<0.05 was considered significant.pDDI: potential drug-drug interaction IQR: interquartile range, APACHE II: acute physiology and chronic health evaluation | ||||
Discussion
In this study, we determined that as the number of medications administered during intensive care stays increased, the potential mild, moderate, and severe drug-drug interactions detected by UpToDate increased. Additionally, an increase in the length of stay in the ICU was associated with an increase in pDDI.
Mild pDDI detected was approximately 10 times more common than moderate pDDI. Mild pDDI has a minimal impact on a patient’s clinic, or medication use is more likely to benefit the patient. Moderate and severe pDDI is more clinically significant and was found to be highest in the patient group who used the most medication.
According to the results of our study, mild and moderate interactions increased with the duration of ICU stay, whereas no clear results were obtained for severe interactions. This situation suggests that even though the patient’s stays is long, physicians are careful not to use drugs together, which may cause serious interactions. However, the possible reasons why pDDIs are less common in the postoperative group are the shorter length of stay and the lower number of medications used. Similarly, the fact that severe pDDI, but not moderate pDDI, was found more frequently in patients with more diseases before ICU admission can be explained by the possibility of using multiple medications in patients with multiple diseases. Another explanation may be that intensive care physicians have low levels of knowledge about the drugs used outside intensive care.
In the study conducted by Uijtendaal et al. (11), who obtained similar results to those obtained in the current study, 54% of the patients were exposed to at least one pDDI on 27% of the hospitalization days. In the same study, the number of days and number of patients exposed to ≥1 pDDI increased in patients with long-term stay in the ICU, high expected mortality rate according to APACHE IV, chronic diseases, and high number of medications used, in patients who received MV support, and who died in intensive care. In addition, similar to our results on the effect of length of stay, Gutiérrez-Valencia et al. (12) found in their study that the number of medications used temporarily during ICU stay increased significantly and PIIE increased due to this increase.
In the meta-analysis conducted by Fitzmaurice et al. (13) to investigate pDDIs in ICUs, it was emphasized that 58% of patients admitted to the ICU may be exposed to at least one pDDI. It has been stated that higher-risk drugs are typically given to critically ill patients compared with other patient populations; therefore, pDDIs may occur between 1 and 10 times per patient. In another meta-analysis, 67% of ICU patients were exposed to at least one pDDI (14). In this study, the daily and total values of mild pDDI were significantly higher than those of moderate and severe pDDI. However, our findings of moderate and severe interactions, which are more clinically significant, were similar to those of Fitzmaurice et al. (13).
Many studies (2, (2, 15, 16), such as Jain et al. (17), have emphasized that as age increases, systemic diseases increase, and the number of drugs used may increase accordingly. It has been observed that the pDDI increases with the number of drugs used. Similar results were obtained in our study.
Rodrigues et al. (18) showed that the duration of intensive care stay was longer in patients with more severe pDDIs. In our study, mild, moderate, and severe pDDIs increased as the duration of intensive care stay increased. Depending on the severity of the critical illness, the length of stay of patients in ICUs may vary, and patients may receive complex treatments during this period. The cause and effect relationship between patients with a high number of pddises and prolonged stay in intensive care is not clear. Although long periods of stay in the ICU increase the risk of pDDI, negative clinical responses caused by pDDI may also prolong the stay of patients in the ICU.
It has been shown that prolonged mechanical ventilation due to sedation, fluid overload, and exceeding therapeutic drug concentrations are less common in ICUs where clinical pharmacologists play an active role (19). Although pharmaceutical care is practiced across many disciplines, critically ill patients require additional evaluation due to the complexity of medication regimens and disease states. We are aware of the importance of clinical pharmacologists as a part of a multidisciplinary team. In addition to all these positive aspects, they can also contribute to the training of intensive care teams.
Medication errors are more common in patients with multiple drug use, long hospital stays, and organ failure. In this regard, we believe that ICUs are very important in terms of treatment planning, administration, monitoring, and evaluation of results. Electronic order systems warn healthcare professionals about the appropriate dosage, correct drug selection, and drug-drug interactions when creating a treatment plan. For this reason, we believe it is useful to use an electronic order system that warns the physician who decides on the treatment and the nurses who apply it to minimize the margin of error in ICUs where a patient-based treatment plan is made.
Although drug combinations causing interactions were not recorded in our study, combinations of combivent and quetiapine and of combivent and carvedilol are the most common combinations that cause severe pDDI. In our study, severe pDDI was detected much less frequently than moderate and mild pDDI. We believe that this may have occurred because physicians are better aware of the contraindicated use of drug combinations.
Some enteral nutrition products and blood products are not available in the database used to detect pDDI. The interactions of the ingredients in these treatments with other drugs were not evaluated.
As a result, in the current study, in the screening performed on critically ill patients with the UpToDate mobile application, it was determined that the PDDI increased as the number of drugs used in the ICU increased, and that there was a relationship between length of stay and pDDI rates.
Ethics
Authorship Contributions
References
- Vanham D, Spinewine A, Hantson P, Wittebole X, Wouters D, Sneyers B. Drug-drug interactions in the intensive care unit: Do they really matter? J Crit Care. 2017;38:97-103.
- Smithburger PL, Kane-Gill SL, Seybert AL. Drug-drug interactions in the medical intensive care unit: an assessment of frequency, severity, and the medications involved. Int J Pharm Pract. 2012;20:402-8.
- Cullen DJ, Sweitzer BJ, Bates DW, Burdick E, Edmondson A, Leape LL. Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med. 1997;25:1289-97.
- Plaza J, Alamo M, Torres P, Fuentes A, López F. Drug interactions and adverse events induced by drugs used in an intensive care unit. Revista medica de Chile. 2010;138:452-60.
- Alvim MM, Silva LA, Leite IC, Silvério MS. Adverse events caused by potential drug-drug interactions in the intensive care unit of a teaching hospital. Rev Bras Ter Intensiva. 2015;27:353-9.
- Ismail M, Khan F, Noor S, Haider I, Haq IU, Ali Z, et al. Potential drug-drug interactions in medical intensive care unit of a tertiary care hospital in Pakistan. Int J Clin Pharm. 2016;38:1052-6.
- Murtaza G, Khan MY, Azhar S, Khan SA, Khan TM. Assessment of potential drug-drug interactions and their associated factors in the hospitalized cardiac patients. Saudi Pharm J. 2016;24:220-5.
- Moura C, Prado N, Acurcio F. Potential drug-drug interactions associated with prolonged intensive care unit stays: a retrospective cohort study. Clin Drug Investig. 2011;31:309-16.
- Monteiro CRA, Schoueri JHM, Cardial DT, Linhares LC, Turke KC, Steuer LV, et al. Evaluation of the systemic and therapeutic effects of drug interactions in oncology patients. Rev Assoc Med Bras (1992). 2019;65:611-7.
- Patel D, Bertz R, Ren S, Boulton DW, Någård M. A systematic review of gastric acid-reducing agent-mediated drug-drug interactions with orally administered medications. Clin Pharmacokinet. 2020;59:447-62.
- Uijtendaal EV, van Harssel LL, Hugenholtz GW, Kuck EM, Zwart-van Rijkom JE, Cremer OL, et al. Analysis of potential drug-drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34:213-9.
- Gutiérrez-Valencia M, Izquierdo M, Malafarina V, Alonso-Renedo J, González-Glaría B, Larrayoz-Sola B, et al. Impact of hospitalization in acute geriatric units on polypharmacy and potentially inappropriate prescriptions: a retrospective study. Geriatrics & gerontology international. 2017;17:2354-60.
- Fitzmaurice MG, Wong A, Akerberg H, Avramovska S, Smithburger PL, Buckley MS, et al. Evaluation of potential drug–drug interactions among adults in the intensive care unit: a systematic review and meta-analysis. Drug Saf. 2019;42:1035-44.
- Zheng WY, Richardson L, Li L, Day R, Westbrook J, Baysari M. Drug-drug interactions and their harmful effects in hospitalized patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74:15-27.
- Cruciol-Souza JM, Thomson JC. Prevalence of potential drug-drug interactions and their associated factors in a Brazilian teaching hospital. J Pharm Pharm Sci. 2006;9:427-33.
- Grenouillet-Delacre M, Verdoux H, Moore N, Haramburu F, Miremont-Salamé G, Etienne G, et al. Life-threatening adverse drug reactions at admission to medical intensive care: a prospective study in a teaching hospital. Intensive Care Med. 2007;33:2150-7.
- Jain S, Jain P, Sharma K, Saraswat P. A prospective analysis of drug interactions in patients of intensive cardiac care unit. J Clin Diagn Res. 2017;11:FC01-4.
- Rodrigues AT, Stahlschmidt R, Granja S, Pilger D, Falcão ALE, Mazzola PG. Prevalence of potential drug-drug interactions in the intensive care unit of a Brazilian teaching hospital. Brazilian Journal of Pharmaceutical Sciences. 2017;53:e16109.
- Askari M, Eslami S, Louws M, Wierenga PC, Dongelmans DA, Kuiper RA, et al. Frequency and nature of drug-drug interactions in the intensive care unit. Pharmacoepidemiol Drug Saf. 2013;22:430-7.
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.