Abstract
Objective
We aimed to compare the advantages and disadvantages of saline (0.9% NACI) and balanced crystalloid (Isolene or Lactated ringer) solutions in patients with diabetic ketoacidosis (DKA).
Materials and Methods
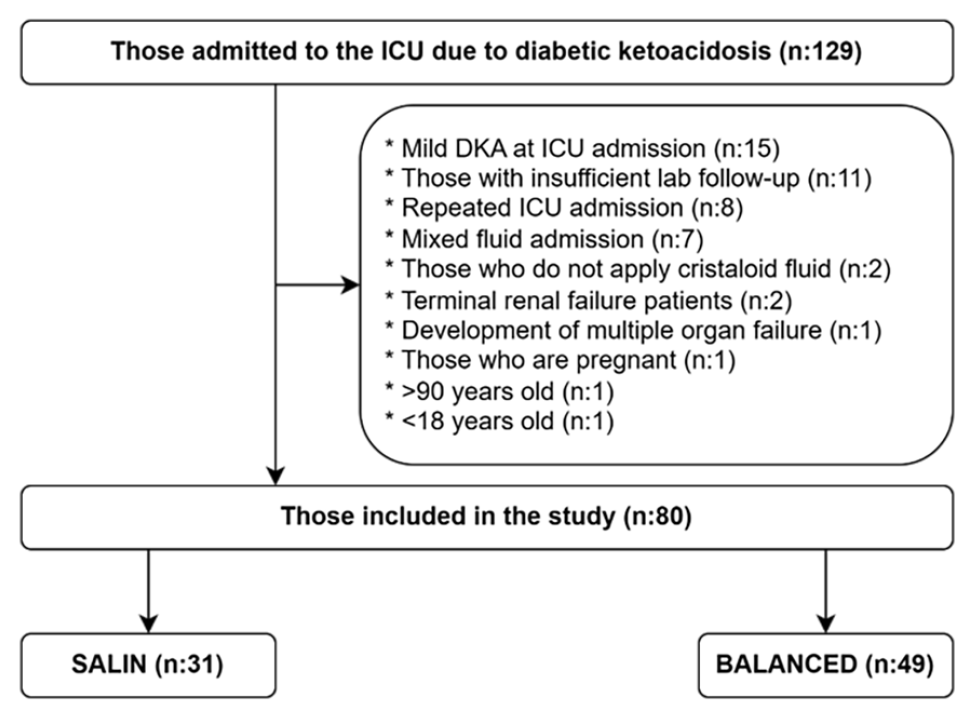
The study was conducted retrospectively on 80 patients (saline=31, balanced=49) with moderate-to-severe DKA among 129 patients with DKA who were admitted to the adult intensive care unit (ICU) between 2013 and 2023.
Results
The DKA resolution times were similar in the saline and balance groups [12 h (6-16), 9 h (7-12), p=0539]. Statistically, the blood chlorine level after DKA resolution was higher in the saline group than in the balanced group (115±5.5, 110.8±4.4, p<0.001) and the anion gap value was lower [5.9 (3.9-10.6), 9.7 (7.0-12.0), p=0.005]. The blood potassium level after DKA solution was lower than normal in the saline group [3.4(3.1-3.6), 3.6(3.2-4.0), p=0.088]. There were no statistically significant differences between the saline and balanced groups in terms of 1-month mortality rates [0(0), 2(4.1), p=0.524], need for renal replacement therapy [1(3.2), 2(4.1), p=1.000], and ICU stay hours [46 (32-70), 44 (36-68), p=0.961].
Conclusion
The choice of saline or balanced crystalloid solution as the initial resuscitation fluid has no effect on DKA resolution time, mortality rate, or ICU length of stay. However, balanced electrolyte solutions have a lower side effect profile.
Keywords: Diabetic ketoacidosis, saline, balanced crystalloid, resolution, mortality
Introduction
Diabetic ketosis (DKA) is a metabolic disorder characterized by hyperglycemia, ketosis, and severe dehydration (due to osmotic diuresis) caused by the absence or deficiency of insulin (1). The frequency of diabetic ketoacidosis varies between 2.8% and 6.3% and is increasing gradually (2, 3). Although DKA can be observed in all age groups, 80% of individuals are over the age of 18 (3). Although DKA is mostly observed in patients with type 1 diabetes (2/3), it can also be observed in patients with type 2 diabetes (4). Although infection is the most common cause of DKA triggering in patients with diabetes mellitus, it can also occur due to events such as not using insulin therapy, trauma, myocardial infarction, cerebrovascular accident, and pancreatitis (3, 5).
Due to the presence of deep metabolic acidosis, DKA treatment is usually performed in intensive care units (ICU) (6). The mainstay of DKA treatment is intravenous (IV) replacement for existing insulin deficiency and fluid loss. Crystalloids are considered superior to colloids in IV fluid replacement (7-9). However, the debate continues as to whether saline (0.9% NaCI) or balanced crystalloid solutions are superior (9,10).
The aim of this study was to investigate the clinical advantages and disadvantages of saline and balanced crystalloid solutions as initial resuscitation fluids in patients admitted to the ICU for moderate to severe DKA.
Materials and Methods
Design and Study Population
Patients admitted to the adult ICU for DKA between 2013 and 2023 were retrospectively evaluated. Among the 129 patients admitted to the ICU, those with mild DKA, recurrent ICU hospitalizations due to DKA, those who had mixed fluid replacement (>1 L intake from the other fluid group), those whose blood gas and electrolyte (Na, K, CI) were not checked every 2-4 hours, and those who were not given crystalloid solutions. Patients with end-stage renal failure, multiple organ failure (MOF), pregnant women, and patients aged 18 years and >90 years were excluded from the study (Figure 1).
These patients were divided into 2 groups, who received saline (0.9% NaCl; pH 5.5) or balanced crystalloid solutions [(Izolen; pH 7.4, Na 140-141 mEq/L, CI 98-103 mEq/L, K 5-10 mEq/L, Acetate 27-47 mEq/L and others) or (Lactated Ringer; pH 6.5, Na 130 mEq/L, CI 98-109 mEq/L, K 4-5 mEq/L, Lactate 27-28 mEq/L and others)] as the first resuscitation fluid during ICU follow-up until DKA resolution.
The study was conducted in full accordance with local Good Clinical Practice Guidelines and current legislation. Ethical approval was obtained from the University of Health Sciences Türkiye, Bakırköy Dr. Sadi Konuk Training and Research Hospital Clinical Research Ethics Committee (decision number: 2023/10, date:22.05.2023).
Protocol
DKA was diagnosed if the following 3 criteria were met:
1.Having a blood glucose level of >250 mg/dL on admission to the hospital or having diabetes mellitus,
2.Having ≥ 2+ ketonuria in urine,
3.Serum HCO3 concentration <15 mmol/L and/or venous Ph<7.3.
Patients with DKA were categorized as mild (serum bicarbonate, 15-18 mEq/L; AG >10; plasma glucose concentration, >250 mg/dL), moderate (serum bicarbonate, 10-15 mEq/L; AG >12; plasma glucose concentration, >250 mg/dL), or severe (serum bicarbonate, <10 mEq/L; AG >12; plasma glucose concentration, >250 mg/dL). AG (Anion GAP) was calculated as follows;
AG = (Na+K)-(CI+HCO3)
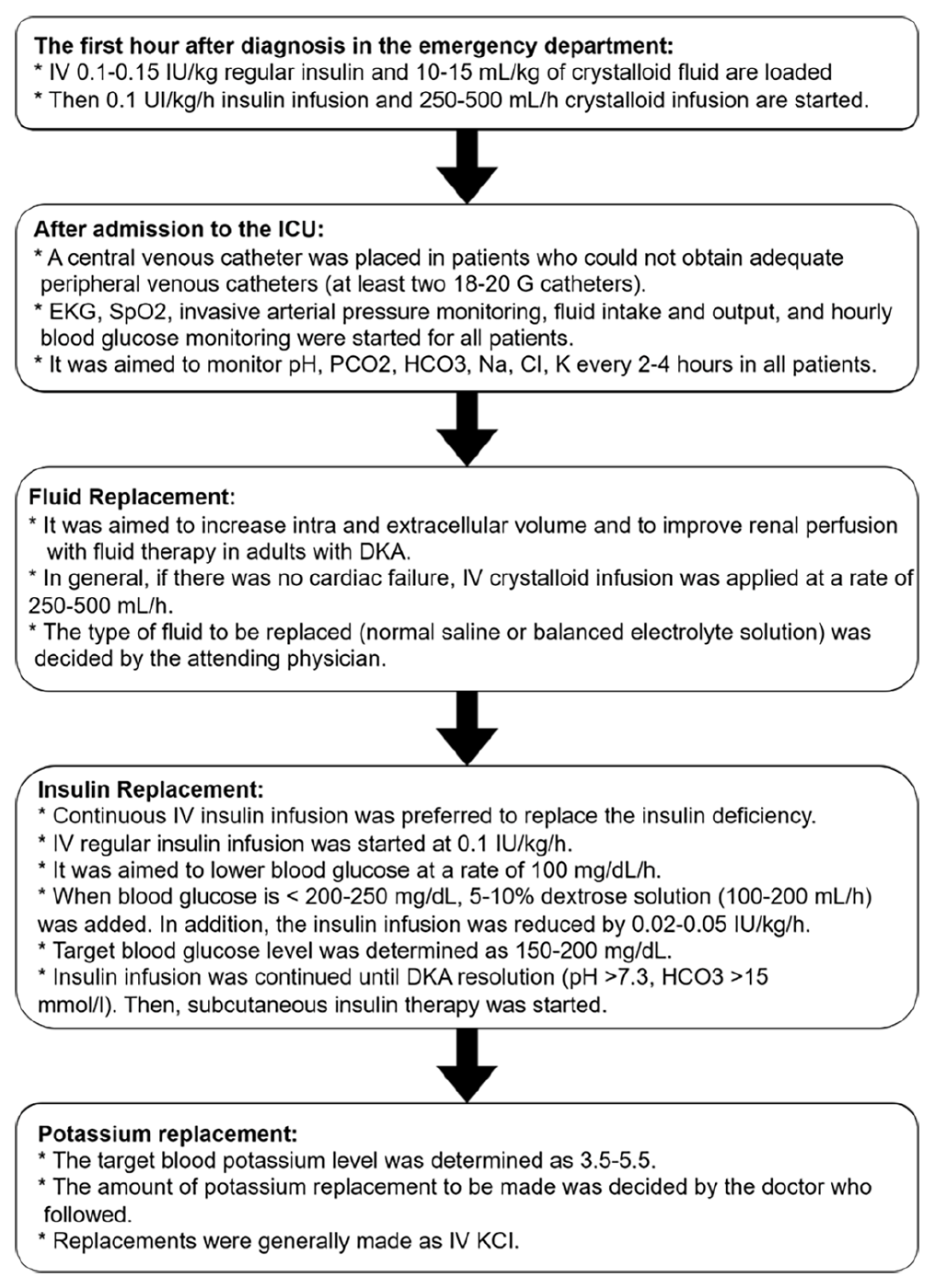
After diagnosis of DKA, IV insulin and fluid loading was performed in the first hour before admission to the ICU in all patients. On admission to the ICU, empirical antibiotic therapy was initiated for patients whose clinical and laboratory parameters were compatible with infection (WBC>20,000 X109/L, C-reactive protein (CRP) >5 mg/L or Procalcitonin>0.5 ng/mL).
The follow-up and treatment algorithm of patients diagnosed with DKA admitted to the ICU is summarized below (Figure 2).
Data Collection
Study data were obtained retrospectively from the ImdSoft-Metavision/QlinICU Clinical Decision Support Software’ system. Age, sex, body mass index (BMI), comorbidities, white blood cell (WBC), hemoglobin, platelets, blood gas (pH, PCO2, HCO3, Base excess, Lactate), glucose, urea, creatinine, total bilirubin, Na, CI, K, CRP, and procalcitonin data were collected for all patients at ICU admission. Again, using these data, CCI (Charlson Comorbidity Index), SOFA (Sequential Organ Failure Assessment), and AKI (Acute Kidney Injury ) scores were calculated (Supplementary Document). The DKA resolution time (pH≥7.3 and HCO3 ≥15) was then determined in all patients. Data on total insulin use, crystalloid solutions (normal saline, balanced crystalloid), 5-10% dextrose solution, and amount of KCl replacement used during this period were collected. Finally, data on total LOS (length of stay) in the ICU, the need for RRT (Renal replacement therapy) and in-hospital 1-month mortality were collected for all patients.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was used to determine whether the data were normally distributed. Categorical variables are given as frequency (n) and percentage (%), numerical variables mean ± standard deviation or median with interquartile range (IQR) Independent-Samples t-test was used to compare quantitative variables with normal distribution between the two groups. Mann-Whitney U test was used for comparisons between two groups of quantitative variables that did not show normal distribution. Pearson’s chi-square, continuity correction, or Fisher’s exact test were used to compare categorical variables. Statistical significance was set as p<0.05.
Results
A total of 80 patients (saline=31, balanced=49) were included in the study. The majority of ICU admissions in both the saline and balanced groups were from the emergency department [n=29(93.5%), n=44(89.8%), p=0.700, respectively,]. Other patients were admitted from external centers and received post-operative or normal in-patient services. There was no statistically significant difference between the saline and balanced crystalloid groups in terms of length of stay (hours) in the emergency department [3.5 (2.0-5.0), 4.0 (2.6-5.8), p>0.077, respectively ] (Table 1).
| Table 1: Demographic and clinical characteristics | |||
|
|
Saline (n=31) |
Balanced (n=49) |
p-value |
|
ICU admission type (ED), n(%) |
29 (93.5) |
44(89.8) |
0.700 |
|
ED duration (h), median (IQR) |
3.5 (2.0-5.0) |
4 (2.6-5.8) |
0.077 |
|
Age, median (IQR) |
35 (21-53) |
27 (20-48) |
0.335 |
|
Female, n(%) |
16 (51.6) |
27 (55.1) |
0.940 |
|
Body mass index, mean ± SD |
23.0±3.1 |
24.6±4.6 |
0.090 |
|
CCI score, median (IQR) |
2 (1-3) |
1 (1-2) |
0.568 |
|
SOFA score, median (IQR) |
1 (0-2) |
1 (0-2) |
0.381 |
|
Type-1 diabetes mellitus, n(%) |
22 (71.0) |
38 (77.6) |
0.691 |
|
Cause of DKA (Infection), n(%) |
22 (71.0) |
33 (67.3) |
0.926 |
|
Severe DKA, n(%) |
17 (54.8) |
37 (75.5) |
0.093 |
|
Admission Lab, median (IQR) |
|
|
|
|
Ph, median (IQR) |
7.15 (7.03-7.25) |
7.13 (7.07-7.20) |
0.607 |
|
PCO2 (mmHg), median (IQR) |
18 (10-22) |
16.9 (11.7-21.4) |
0.953 |
|
HCO3 (mmol/L), median (IQR) |
9 (6.5-11.2) |
8.2 (7.1-9.8) |
0.499 |
|
Base excess (mmol/L), mean ± SD |
-21.6±5.6 |
-22.7±4.4 |
0.336 |
|
NA (mmol/L), median (IQR) |
134 (132-137) |
134 (131-137) |
0.886 |
|
K (mmol/L), median (IQR) |
4.6 (4.2-5.3) |
4.5 (3.9-5.0) |
0.254 |
|
CI (mmol/L), mean ± SD |
102.5±8.4 |
102.0±6.7 |
0.757 |
|
Anion gap, median (IQR) |
24.8 (21.6-30.1) |
26.8 (23.0-30.3) |
0.412 |
|
Lactate (mmol/L), median (IQR) |
1.6 (1.2-2.8) |
1.4 (1.2-2.3) |
0.583 |
|
Glukoz (mg/dL), median (IQR) |
360 (268-466) |
281 (240-351) |
0.082 |
|
Urea (mg/dL), median (IQR) |
38 (31-54) |
30.3 (19.3-50.0) |
0.091 |
|
Creatınıne (mg/dL), median (IQR) |
0.95 (0.79-1.18) |
0.89 (0.73-1.13) |
0.716 |
|
Total Bilirubin (mg/dL), median (IQR) |
0.32 (0.2-0.5) |
0.25 (0.18-0.44) |
0.534 |
|
CRP(mg/L), median (IQR) |
10.5 (1.95-43.75) |
13.5 (5.2-56.0) |
0.474 |
|
Procalcitonin (ng/ml), median (IQR) |
0.5 (0.2-2.5) |
0.75 (0.27-3.74) |
0.537 |
|
Hemoglobin (g/dL), median (IQR) |
12.4 (10.9-13.3) |
12.7 (11.0-13.7) |
0.448 |
|
Platelet (X109/L), mean ± SD |
300±133 |
297±120 |
0.904 |
|
WBC (X109/L), mean ± SD |
17.6±5.8 |
18±7.9 |
0.824 |
|
AKI, n(%) |
14 (45.2) |
20 (40.7) |
0.637 |
|
AKI-1 |
12 (38.7) |
18 (36.7) |
|
|
AKI-2 |
2 (6.5) |
2 (2.0) |
|
|
AKI-3 |
0 (0) |
1 (2.0) |
|
| ED: emergency department, CCI: Charlson comorbidity index, SOFA: sequential organ failure assessment, DKA: diabetic ketoacidosis, CRP: C-reactive protein, WBC: white blood cells, AKI: acute kidney injury | |||
There were no statistically significant differences between the saline and balanced crystalloid groups in terms of age, gender, and BMI (p=0.335, p=0.940, p=0.090, respectively,). There were no statistically significant differences between the saline and balanced crystalloid groups in terms of the CCI and SOFA mortality score (p=0.568, p=0.381, respectively). DKA was most common in type 1 diabetes in both the saline and balanced crystalloid groups 22 (71.0), 38 (77.6), p=0.691, respectively]. The most common cause of DKA in both the saline and balance crystalloid groups was infection [22(71.0), 33(67.3), p=0.926, respectively]. There was no statistically significant difference between the saline and balanced crystalloid groups in terms of DKA severity (p=0.093). There was no statistically significant difference between the saline and balanced crystalloid groups in terms of the rate of development of AKI due to DKA 14 (45.2%), 20 (40.7%), p=0.637, respectively]. There were no statistically significant differences between the saline and balanced crystalloid groups in terms of ICU admission laboratory parameters (p>0.05) (Table 1).
Although DKA resolution time was higher in the saline group, there was no statistical difference with balanced crystalloid solution 12 (6-16), 9 (7-12), p=0.539, respectively]. The amounts of total insulin, fluids and 5-10% dextrose solutions used in IV therapy were similar in both groups (p=0.921, p=0.693, p=0.932, respectively). There was no statistically significant difference between the saline and balanced crystalloid groups in terms of the number of patients given KCI and amount of KCI replacement (p=1.000, p=0.331, respectively) (Table 2).
| Table 2: Treatment outcomes of patients receiving saline and balanced crystalloid solutions | |||
|
|
Saline (n=31) |
Balanced (n=49) |
p-value |
|
DKA resolution time(Hour), median (IQR) |
12 (6-16) |
9 (7-12) |
0.539 |
|
IV replacements therapies, median (IQR) |
|||
|
Total insulin, IU |
40 (26-64) |
42 (28-56) |
0.921 |
|
Total dextrose (5-10%), L |
1 (1-2) |
1 (1-2) |
0.932 |
|
Total fluid, L |
4 (2.0-7.0) |
3.5 (3.0-5.3) |
0.693 |
|
KCI, mEq |
40 (40-90) |
50 (50-100) |
0.331 |
|
Number of patients given KCI, n(%) |
4 (12.9) |
7 (14.3) |
1.000 |
|
After resolution lab |
|||
|
Ph, median (IQR) |
7.35 (7.33-7.38) |
7.34 (7.31-7.38) |
0.232 |
|
PCO2 (mmHg), median (IQR) |
27.8 (25.5-31.0) |
28.3 (26.0-33.5) |
0.390 |
|
HCO3 (mmol/L), median (IQR) |
17.0 (16-18) |
17.3 (16-19) |
0.317 |
|
Base excess (mmol/L), mean ± SD |
-9.22±2.2 |
-8.58±2.8 |
0.283 |
|
Anion gap, median (IQR) |
5.9 (3.9-10.6) |
9.7 (7.0-12.0) |
0.005* |
|
Na (mmol/L), median (IQR) |
135 (132-139) |
134 (131-137) |
0.454 |
|
K (mmol/L), median (IQR) |
3.4 (3.1-3.6) |
3.6 (3.2-4.0) |
0.088 |
|
CI (mmol/L), mean ± SD |
115.0±5.506 |
110.8±4.4 |
<0.001* |
|
RRT need, n(%) |
1 (3.2) |
2 (4.1) |
1.000 |
|
LOS in ICU (Hour), median (IQR) |
46 (32-70) |
44 (36-68) |
0.961 |
|
Mortality, n(%) |
0 (0) |
2 (4.1) |
0.524 |
| *p<0.05, DKA: diabetic ketoacidosis, IV: intravenous, Lab: laboratory, RRT: renal replacement therapy, LOS: length of stay, ICU: intensive care unit | |||
There was statistically significant difference between saline group and balanced crystalloid group in terms of the blood chlorine level after DKA resolution (115±5.5, 110.8±4.4, p<0.001, respectively). There was statistically significant difference between saline group and balanced crystalloid group in terms of the anion gap value after DKA resolution [5.9 (3.9-10.6), 9.7 (7.0-12.0), p=0.005, respectively]. There was no statistically significant difference between the saline and balanced crystalloid groups in terms of blood potassium levels after DKA resolution [3.4 (3.1-3.6), 3.6 (3.2-4.0), p=0.088, respectively]. There were no statistically significant differences between the saline and balanced crystalloid groups in terms of blood pH, PCO2, HCO3, base excess, and sodium levels after DKA resolution (p>0.05) (Table 2).
There was no statistically significant difference between the saline and balanced groups as the first resuscitation fluid in terms of mortality, LOS in the ICU, and RRT (p=0.524, p=0.961 p=1.000, respectively) (Table 2).
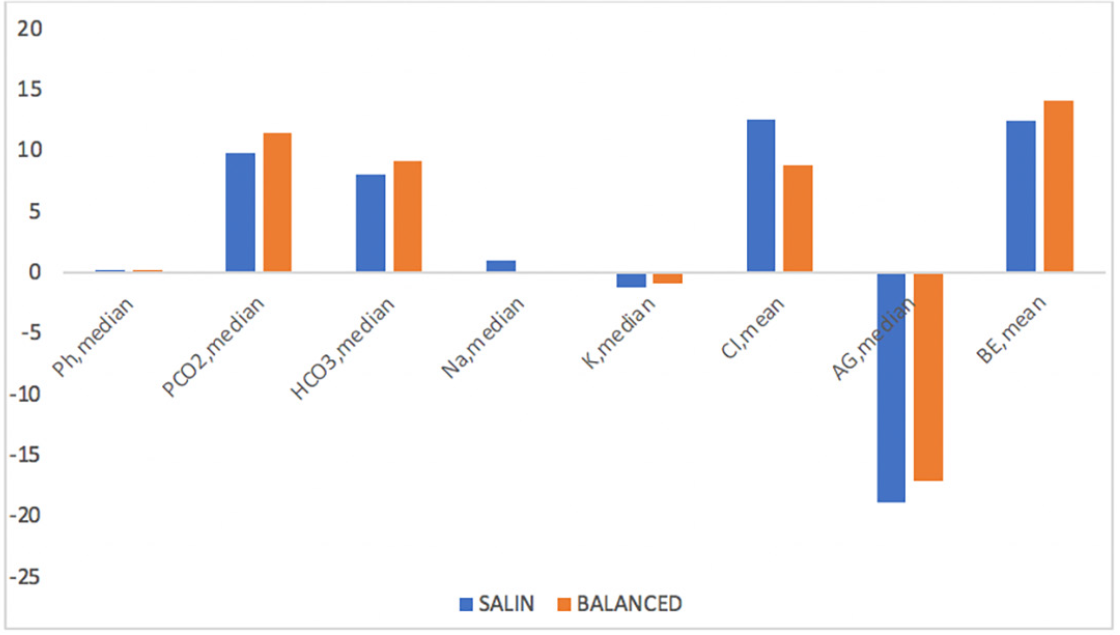
The range of increases in blood CI levels and decreases in the amount of anion gap were more pronounced in the saline group than in the balanced group. On the other hand, the ranges of improvement in blood PCO2, HCO3, and base excess values were lower in the saline group. The range of changes in other laboratory parameters (pH, Na, K) was similar between the groups (Figure 3).
Discussion
We conducted this study to determine the advantages and disadvantages of saline and balanced crystalloid solutions as the initial resuscitation fluid in patients developing DKA. We did not detect any differences between saline and balanced crystalloid solutions in terms of DKA resolution time, 1-month mortality rate, and ICU length of stay. At the same time, the choice of saline or balanced electrolyte solution did not change the total amount of insulin used. In two prospective randomized controlled trials in 2011 and 2012 comparing the use of saline and balanced crystalloid solutions in the treatment of DKA, no superiority of either crystalloid solution was found (10-12). In a retrospective study of 85 patients in the emergency department in 2018, no difference was found in the time to resolution of DKA with the choice of crystalloid solution (13). Subsequently, in a post hoc secondary subgroup analysis of 172 patients that included 2 randomized controlled trials on emergency room and ICU patients in 2020, balanced crystalloid solution therapy was associated with faster resolution of DKA (14). Finally, in a meta-analysis of 8 randomized controlled trials involving a total of 482 patients comparing saline and balanced crystalloid solutions in 2022, it was found that the use of saline caused a slight increase in the risk of DKA resolution time and hospital stay compared with the use of balanced crystalloid solutions (1). In our study, the DKA resolution time was longer in patients receiving saline therapy, but this difference was not statistically significant. When these studies are evaluated together, no evidence that saline solutions are superior to balanced crystalloid solutions has been presented. On the contrary, a significant number of these studies showed that the use of saline can lead to hyperchloremic acidosis and prolonged DKA resolution time.
In our study, when DKA resolution was achieved, an increase in the blood chlorine level was observed in both groups. However, the increase in the blood CI level range was much more pronounced in the saline group than in the balanced group. At the same time, the range of decrease in the anion gap content was much more pronounced in the saline replacement group. On the other hand, the range of recovery of blood PCO2, HCO3, and base deficit was lower in the saline group. Studies have shown that hyperchloremic acidosis, low anion gap, and renal HCO3 loss may develop due to rapid and high-volume IV infusion of high-volume acidic saline solution (1, 15). Therefore, although DKA regresses with insulin replacement in the saline replacement group, metabolic acidosis due to hyperchloremia may develop. In addition, although the duration of DKA resolution was longer in the saline group, the amount of HCO3 increase and the range of base excess recovery amount may have been lower. It was observed that hyperchloremia developed in the balanced group but not in the saline group. This may be due to the use of saline solution to replace insulin, potassium, and other IV drugs.
The number and amount of patients who received potassium replacement were similar between the groups. The potassium level measured after DKA resolution was lower in the saline group but within the lower limits in both groups. When DKA develops due to insulin deficiency, potassium levels tend to decrease intracellularly and increase extracellularly (3). Later, with the initiation of insulin therapy, hypokalemia may develop due to the shift of potassium into the cell (4). Therefore, potassium must be replaced. The low potassium levels measured after resolution of DKA in our patient population, especially in the saline group, suggest that potassium replacement was inadequate.
In both patient groups, the proportion of patients who developed AKI upon admission to the hospital was similar. AKI may develop due to renal perfusion impairment, as well as deterioration in all tissues due to severe volume deficit due to osmotic diuresis. High-volume replacement is needed for the treatment of AKI (16). However, there are concerns that renal vasoconstriction and decreased glomerular filtration rate may occur due to hyperchloremia associated with saline infusion (15, 17). In our study, although the AKI rates were high in both groups upon admission, the need for RRT was similarly low. In a study evaluating 15,802 critically ill patients hospitalized in multicentric ICU in 2018, no statistically significant difference was found between the use of saline or balanced crystalloid solutions, the need for new RRT, and the rate of development of permanent renal dysfunction (18).
Both patient groups consisted mostly of young patients who did not have any additional comorbidities other than diabetes mellitus. Therefore, CCI scores were low in both groups. The SOFA scores used to predict mortality were low in both patient groups. The low SOFA score was consistent with the low overall mortality rate. Although SOFA scores were low, most patients in both patient groups had severe DKA.
Patients with type 1 diabetes mellitus constituted the majority of the patients. Although DKA can be observed in type 2 diabetes mellitus due to insulin resistance, it is most likely to occur in type 1 diabetes mellitus, which mainly develops due to insulin insufficiency (4, 6). In our study, as in the literature, the most common cause of DKA in both patient groups was infection (3, 5). Correspondingly, both patient groups had higher WBC count, CRP level, and procalcitonin level.
The current study has several limitations: Firstly, the study was retrospective. Due to the retrospective nature of the study, some patients who were not followed up frequently and in accordance with the study protocol were excluded from the study. However, considering the original studies on DKA, it was important to ensure that a significant number of patients with DKA were examined. Second, the study was single-centered. Third, although the amount of intravenous insulin and crystalloid loading administered within the first hour after DKA diagnosis is standardized, the lack of recorded data on the exact amount of treatments administered during the period until ICU admission is an important limitation. The mean length of stay in the emergency room was similar between the groups. Although the mean length of stay in the emergency department before ICU admission was similar in both groups, we did not include the treatment administered in the emergency department in our evaluation of both patient groups. We planned to compare the treatment after ICU admission.
Conclusion
The saline was superior to the balanced crystalloid solution as the initial resuscitation fluid in patients with DKA. On the contrary, rapid and high-volume saline solution use can lead to the development of hyperchloremic metabolic acidosis. Greater attention should be paid to adequate potassium replacement regardless of whether a saline solution or a balanced solution is used. In addition, potassium replacement with potassium phosphate is more appropriate for preventing hyperchloremia.
However, no effect of selecting saline or balanced crystalloid solution on mortality and ICU stay was observed. The advantages of saline solutions, such as their cost and ease of supply, may make them a reason for centers with limited resources. However, for DKA treatment, we recommend the use of balanced crystalloid solutions as the first choice because they have a lower side effect profile.
Click the link to access Supplementary Document: https://l24.im/trF8
Ethics
Authorship Contributions
References
- Alghamdi NA, Major P, Chaudhuri D, Tsui J, Brown B, Self WH, et al. Saline compared to balanced crystalloid in patients with diabetic ketoacidosis: a systematic review and meta-analysis of randomized controlled trials. Crit Care Explor. 2022;4:e0613.
- Sheikh GA, Muhammad D. Frequency of diabetic ketoacidosis in diabetic patients. J. Univ. Med. Dent. Coll. 2011;2:22-7.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335-43.
- Galm BP, Bagshaw SM, Senior PA. Acute management of diabetic ketoacidosis in adults at 3 teaching hospitals in canada: a multicentre, retrospective cohort study. Can J Diabetes. 2019;43:309-315.e2.
- Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:2739-48.
- Mendez Y, Surani S, Varon J. Diabetic ketoacidosis: treatment in the intensive care unit or general medical/surgical ward? World J Diabetes. 2017;8:40-4.
- Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;(2):CD000567.
- Reinhart K, Perner A, Sprung CL, Jaeschke R, Schortgen F, Groeneveld AB, et al. Consensus statement on colloid volume therapy in critically ill patients. Intensive Care Med. 2012;38:368-83.
- Dhatariya KK; The Joint British Diabetes Societies for Inpatient Care. The management of diabetic ketoacidosis in adults—An updated guideline from the Joint British Diabetes Society for Inpatient Care. Diabet Med. 2022;39:e14788.
- Dhatariya KK. Diabetic ketoacidosis. Br Med J. 2007;334:1284-5.
- Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29:670-4.
- Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis--Ringer’s lactate versus normal saline: a randomized controlled trial. QJM. 2012;105:337-43.
- Oliver WD, Willis GC, Hines MC, Hayes BD. Comparison of plasma lyte a and sodium chloride 0.9% for fluid resuscitation of patients with diabetic ketoacidosis. Hosp Pharm. 2018;53:326-30.
- Self WH, Evans CS, Jenkins CA, Brown RM, Casey JD, Collins SP, et al. Pragmatic critical care research group. clinical effects of balanced crystalloids vs saline in adults with diabetic ketoacidosis: a subgroup analysis of cluster randomized clinical trials. JAMA Netw Open. 2020;3:e2024596.
- Eisenhut, M. Causes and effects of hyperchloremic acidosis. Crit Care. 2006;10:413.
- Aditianingsih D, Djaja AS, George YWH. The effect of balanced electrolyte solution versus normal saline on the prevention of hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis: A randomized controlled trial. Medical Journal of Indonesia. 2017;26:134-40.
- Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. 1989;256:F152-7.
- Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829-39.
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.