Abstract
We described a case of a previously healthy young man with Plasmodium vivax induced severe rhabdomyolysis with a creatinine kinase (CK) level of 812,000 U/L leading to acute respiratory failure and subsequent weaning failure. Mix infections with Plasmodium falciparum were ruled out by polymerase chain reaction (PCR) and other causes including trauma, heat exhaustion, autoimmune diseases, inflammatory myopathy, drugs, and infections such as leptospirosis and COVID-19 were excluded. He presented with respiratory distress requiring intubation and ventilatory support. There was no heart or lung pathology, fever, metabolic acidosis, anaemia, or drop in consciousness level upon presentation. Extubation was attempted twice during the first week of admission, however, respiratory failure ensued after each attempt requiring reintubation in which one of the episodes was complicated by lung collapse. The respiratory distress upon presentation and failed extubation episodes were attributed to respiratory muscle weakness secondary to severe rhabdomyolysis. He was successfully extubated after almost two weeks of admission. Despite the extremely high CK level, renal function was unexpectedly preserved without the need for renal replacement therapy. To the best of our knowledge, this is the first reported case of severe rhabdomyolysis induced by P. vivax leading to respiratory failure but with preserved renal function. This case highlights that P. vivax infection can cause severe rhabdomyolysis and consequently acute respiratory failure due to muscle weakness. Awareness of such complications will guide clinicians’ decisions for timely initiation and weaning from mechanical ventilation, hence avoidance of associated complications.
Keywords: rhabdomyolysis, Plasmodium Vivax, respiratory insufficiency, ventilator weaning
Introduction
Rhabdomyolysis as a complication of malaria infection is typically associated with Plasmodium falciparum but is rarely linked to Plasmodium vivax (1). While severe rhabdomyolysis commonly results in life-threatening acute renal failure (2), its association with acute respiratory failure is uncommon (3,4). This report presents a case of rhabdomyolysis due to P. vivax infection leading to acute respiratory failure requiring ventilatory support and subsequent weaning failure, despite preserved renal function.
Case Report
A 24-year-old Bangladeshi male with no known medical history presented to the emergency department with a 5-day history of worsening abdominal and chest pain, progressive shortness of breath, a 2-day history of dark-coloured urine and a 1-month history of intermittent fever and abdominal discomfort. He denied any recent medication use, illicit drugs intake, or recent travel. There were no episodes of vomiting, diarrhoea, jaundice, or weight loss.
On examination, he was alert but profusely diaphoretic, afebrile, tachypnoeic (respiratory rate: 34 breaths/minute), hypertensive (BP: 160/111) and tachycardic (HR: 115 beats/minute). Oxygen saturation was 100% on room air. Chest X-ray was unremarkable. Abdominal examination revealed generalised tenderness with hepatosplenomegaly; the abdomen was soft, non-distended, and without guarding.
Initial biochemical analysis was notable for severe rhabdomyolysis, with markedly elevated creatinine kinase (CK) at 812,000 U/L, CKMB >2,000 ng/ml, aspartate transaminase (AST) 12,593 U/L, alanine transaminase (ALT) 4,361 U/L and lactate dehydrogenase (LDH) 9,945 U/L (Table 1). Urine spectrometry was positive for myoglobin and haemoglobin. Renal function remained within normal limits. Other relevant tests included mildly elevated total bilirubin, negative hepatitis B/C and HIV serologies, low serum paracetamol level, and a negative COVID-19 rapid test. Troponin I was 11 ng/L with no ischaemic changes on ECG. The Leptospira agglutination test was negative.
| Hb = haemoglobin, WBC = white blood cell, Plt = platelet, Na = sodium, K = potassium, Ur = urea, Cr = creat, Alb = albumin, TB = total bilirubin, ALT = alanine transaminase, ALP = alkaline phosphatase, AST = aspartate transamina. | |||||||||||||||
| Table 1. Summary of investigation result throughout admission | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Hb (g/dL) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| WBC (103/uL) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Plt (103/uL) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Na (mmol/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| K (mmol/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ur (mmol/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cr (umol/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CK (U/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| TB (umol/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ALT (U/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ALP (U/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| AST (U/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Given the presence of hepatosplenomegaly and his country of origin, malaria was suspected. Peripheral blood film microscopy confirmed P. vivax infection with a parasite count of 1,670/59 µL. Glucose-6-phosphate dehydrogenase (G6PD) was normal, and treatment with intravenous artesunate and oral primaquine was initiated per local protocol. There was no evidence of anaemia or thrombocytopenia on admission.
He was admitted to the intensive care unit (ICU) for respiratory support with high-flow nasal cannula (60L/min, FiO2 0.4) to reduce the work of breathing. Aggressive intravenous fluid resuscitation and urine alkalinisation were initiated for rhabdomyolysis. N-acetylcysteine (NAC) infusion was started due to transaminitis. Despite these measures, his respiratory distress worsened, and he required intubation after 20 hours in the ICU. This occurred in the absence of hypoxia, sepsis, pulmonary findings, or positive fluid balance. On day 2, malarial polymerase chain reaction (PCR) confirmed mono-infection with P. vivax (parasite count: 0/90 µL). On day 3, he developed acute haemolytic anaemia, with haemoglobin decline and elevated reticulocytes count (5.9%). Peripheral blood smear confirmed haemolysis; iron study was normal.
An initial attempt to extubation on day 5 to Venturi mask (FiO2 60%) failed within hours due to hypercapnic respiratory failure (arterial blood gas: pH 7.17, PaCO2 95 mmHg, PaO2 142 mmHg, bicarbonate 27.6, SaO2 99%), necessitating reintubation. A second extubation on day 8 was followed by desaturation after sips of clear fluid. Chest X-ray revealed total right lung collapse, and urgent bronchoscopy identified presence of a large amount of thick secretions occluding the right bronchial tree. Following the second failed extubation, respiratory muscle weakness was suspected. Neurological examination revealed proximal (shoulder girdle) weakness with otherwise normal findings. Intravenous immunoglobulin (IVIG) was initiated empirically for possible inflammatory myositis. Myositis antibody panel, ANA, complement levels (C3/C4), thyroid function, and HbA1c were all within normal limits. Cultures of blood, urine, and tracheal aspirate were negative.
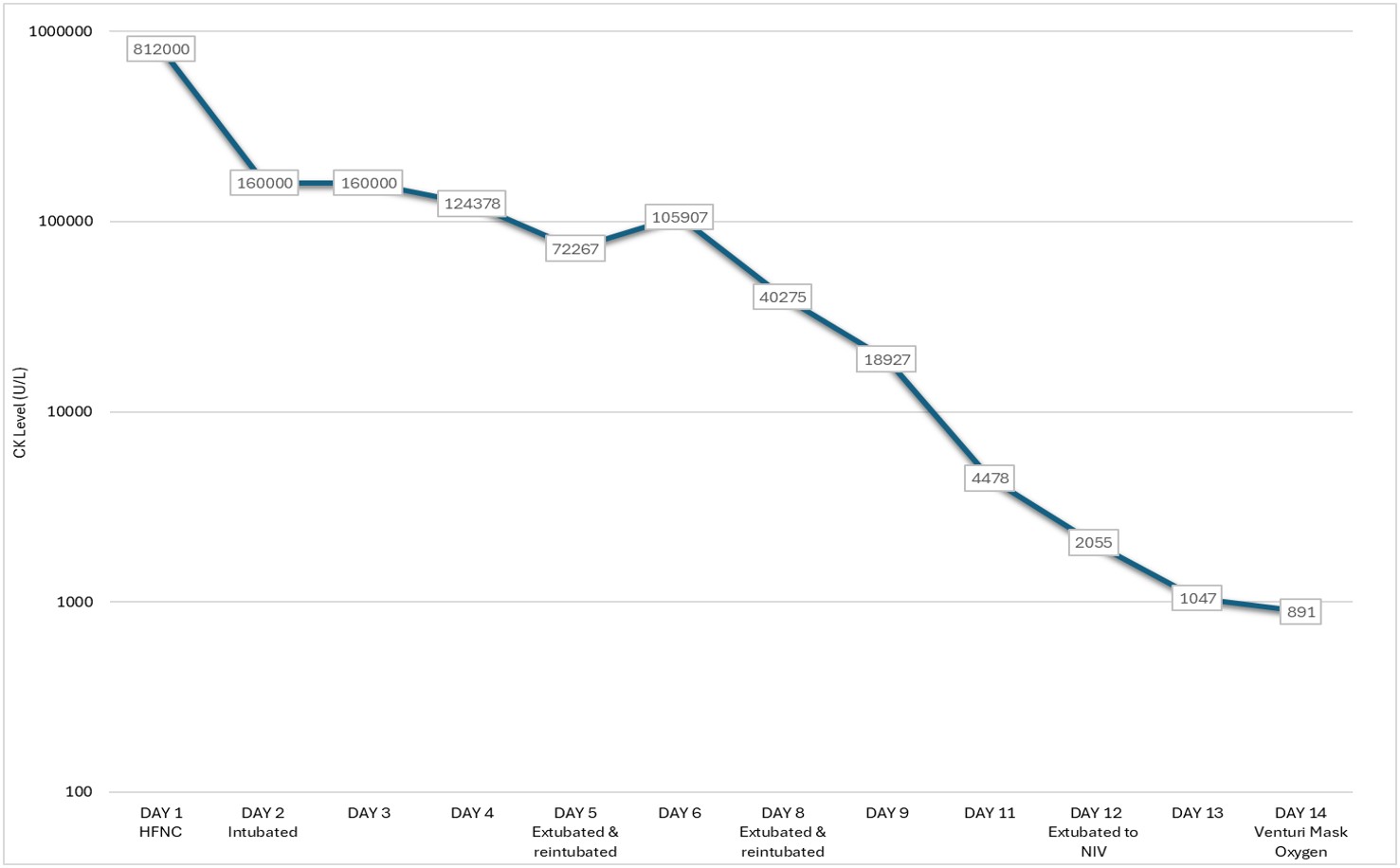
His CK level declined progressively with supportive therapy, and his respiratory status improved in parallel. Successful extubation was achieved once CK level has dropped significantly from peak values (Figure 1). Renal function remained preserved throughout, and renal replacement therapy was not required. Repeat blood films confirmed parasitic clearance. He was extubated to non-invasive ventilation (NIV) on day 12 and subsequently transitioned to conventional oxygen therapy. He was discharged well on day 15 of hospitalisation.
Discussion
P. vivax is the most prevalent species of malaria outside sub-Saharan Africa (5). While historically considered to cause benign disease, emerging evidence demonstrated that P. vivax infection can result in significant morbidity and mortality (6). Rhabdomyolysis, characterised by the breakdown of skeletal muscle with the release of myoglobin, sarcoplasmic proteins, and electrolytes into the circulation (7), is a rare complication of malaria and has been primarily reported in association with P. falciparum (1). To date, only two cases of P. vivax-associated rhabdomyolysis have been reported in literature. One occurred in a patient with myoadenylate deaminase deficiency (MADD) (8). Although muscle biopsy was not performed in our patient to exclude MADD, the absence of hallmark features such as exercise intolerance fatigability, and recurrent myalgia (9), along with its low prevalence (1-2%) in Caucasians (10), makes it unlikely in our Bangladeshi patient.
The second case reported rhabdomyolysis exacerbated by anti-malarial treatment (primaquine and chloroquine) (11). In contrast, our patient presented with markedly elevated CK levels (almost 300 times higher than the second reported case), prior to the initiation of any antimalarial therapy, suggesting that P. vivax infection itself was the likely trigger, confirmed by PCR. Other common causes such as trauma, heat exhaustion, autoimmune diseases, inflammatory myopathy, drugs, and other relevant infections were excluded, further supporting this hypothesis.
Interestingly, while both previously reported cases progressed to acute kidney injury requiring renal replacement therapy, our patient’s renal function remained preserved despite the extremely elevated CK. Early aggressive intravenous hydration, urine alkalinisation, and the patient’s young age may have played a protective role (12). Additionally, we hypothesise that the administration of NAC—initiated for presumed malarial hepatopathy based on marked transaminase elevation (13), may have contributed to renal protection. NAC possesses antioxidant properties and has been shown in preclinical studies to attenuate rhabdomyolysis-induced renal injury, although human data remain limited (14).
While renal involvement is a well-recognised complication of severe rhabdomyolysis, respiratory failure secondary to muscle involvement is less frequently reported and often underappreciated. As illustrated in this case, the need for mechanical ventilation and two failed extubation attempts were attributed to respiratory muscle weakness, despite normal pulmonary auscultation, absence of pneumonia or acute respiratory distress syndrome, and preserved neurological status at the time of ICU admission.
At the time of initial intubation, there were no signs of hypoxaemia, altered mental status, metabolic acidosis, sepsis, or significant anaemia—commonly reported contributors to respiratory distress in malaria (15). During both extubation attempts, the patient had passed spontaneous breathing trials, had a Richmond and Agitation-Sedation Scale (RASS) score of +1, was haemodynamically stable, and not on sedative agents. Pulmonary mechanics and secretion burden were considered acceptable. Nonetheless, extubation failed. First was due to hypercapnic respiratory failure, and later due to a right lung collapse, the latter attributed to retained secretions. Neurological examination performed after the second failed extubation revealed proximal weakness with preserved distal strength and no bulbar deficits. Although myositis panel was negative, empirical IVIG was administered in case of inflammatory myopathy. No infectious, autoimmune, or metabolic contributors were identified.
There are limited reports of rhabdomyolysis from other infectious causes leading to respiratory failure (3,4), but to our knowledge, this is the first report of P. vivax-associated rhabdomyolysis presenting with respiratory muscle weakness and failure requiring prolonged mechanical ventilation. The pathogenesis may involve systemic inflammation, oxidative stress, and microvascular sequestration of infected red cells in muscle capillaries (16). A direct cytopathic effect of P. vivax on muscle tissue has also been postulated (11).
Importantly, myalgia—often absent in up to 50% of rhabdomyolysis cases (17) was not reported by our patient. This may lead clinicians to underestimate the extend of muscle involvement, including respiratory musculature. In our case, overt weakness only became apparent upon closer examination following failed extubation attempt. Anticipating respiratory failure in patients with severe rhabdomyolysis can guide decisions around timing of extubation. Literature suggests muscle regeneration typically begins within 3-5 days post-injury and peaks around 2 weeks (18). Extubation attempts on days 5 and 8 may have been premature, whereas sustained success was achieved only nearly two weeks, aligning with expected recovery time.
Conclusion
P. vivax infection can present with severe rhabdomyolysis and respiratory failure due to muscle weakness, even in the absence of renal impairment. Clinician awareness of this rare but significant complication is crucial, as early recognition may inform ventilator management and optimise extubation timing, ultimately improving patient outcomes.
Acknowledgement
The authors would like to thank the Director General of Health for his permission to publish the paper.
Ethical approval
This study has been approved by the Ministry of Health Malaysia Medical Research & Ethics Committee (approval date: April 3, 2024, number: 24-00632-AX7). Written informed consent was obtained from the participants.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Davis TM, Pongponratan E, Supanaranond W, et al. Skeletal muscle involvement in falciparum malaria: biochemical and ultrastructural study. Clin Infect Dis. 1999;29:831-5. https://doi.org/10.1086/520444
- Candela N, Silva S, Georges B, et al. Short- and long-term renal outcomes following severe rhabdomyolysis: a French multicenter retrospective study of 387 patients. Ann Intensive Care. 2020;10:27. https://doi.org/10.1186/s13613-020-0645-1
- Sotirios K, Vasiliki N, Stamatoula T, Alexia A, Nikolaos M, Sylvia R. A case of rhabdomyolysis and weaning failure in a patient with severe SARS CoV-2 infection. J Acute Med. 2023;13:75-8. https://doi.org/10.6705/j.jacme.202306_13(2).0004
- Gindre H, Féasson L, Auboyer C, Cathébras P. Severe rhabdomyolysis associated with a primary cytomegalovirus infection in an immunocompetent patient. BMJ Case Rep. 2013;2013:bcr2012008140. https://doi.org/10.1136/bcr-2012-008140
- World Health Organization (WHO). Fact sheet about malaria. WHO; 2024. Available at: https://www.who.int/news-room/fact-sheets/detail/malaria (Accessed on Apr 19, 2024).
- Kute VB, Goswami JG, Vanikar AV, et al. Unusual presentation of Plasmodium vivax: a neglected human malaria parasite. Parasitol Res. 2012;110:2573-6. https://doi.org/10.1007/s00436-011-2776-7
- Stanley M, Chippa V, Aeddula NR, Quintanilla Rodriguez BS, Adigun R. Rhabdomyolysis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Poels PJ, Dolmans WM, Gabreëls FJ. Rhabdomyolysis associated with malaria tertiana in a patient with myoadenylate deaminase deficiency. Trop Geogr Med. 1993;45:83-6. https://pubmed.ncbi.nlm.nih.gov/8511818/
- Teijeira S, San Millán B, Fernández JM, et al. Myoadenylate deaminase deficiency: clinico-pathological and molecular study of a series of 27 Spanish cases. Clin Neuropathol. 2009;28:136-42. https://doi.org/10.5414/npp28136
- Sabina RL. Myoadenylate deaminase deficiency. A common inherited defect with heterogeneous clinical presentation. Neurol Clin. 2000;18:185-94. https://doi.org/10.1016/s0733-8619(05)70184-5
- Siqueira AM, Alexandre MA, Mourão MP, et al. Severe rhabdomyolysis caused by Plasmodium vivax malaria in the Brazilian Amazon. Am J Trop Med Hyg. 2010;83:271-3. https://doi.org/10.4269/ajtmh.2010.10-0027
- Hansrivijit P, Yarlagadda K, Puthenpura MM, Cunningham JM. Extremely high creatine kinase activity in rhabdomyolysis without acute kidney injury. Am J Case Rep. 2020;21:e924347. https://doi.org/10.12659/AJCR.924347
- Shoukier H, Dham S, Bergasa NV, Kochar DK, Sirohi P, Abhishek K. Acute hepatitis in malaria. Gastroenterol Hepatol (N Y). 2006;2:35-8.
- Panizo N, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Moreno JA. Molecular mechanisms and novel therapeutic approaches to rhabdomyolysis-induced acute kidney injury. Kidney Blood Press Res. 2015;40:520-32. https://doi.org/10.1159/000368528
- Taylor WR, Hanson J, Turner GD, White NJ, Dondorp AM. Respiratory manifestations of malaria. Chest. 2012;142:492-505. https://doi.org/10.1378/chest.11-2655
- Siagian FE. Malarial related myopathies: a rhabdomyolysis story. Int J Pathogen Res. 2020;5:39-51. https://doi.org/10.9734/ijpr/2020/v5i330137
- Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-56. https://doi.org/10.1515/CCLM.2010.151
- Laumonier T, Menetrey J. Muscle injuries and strategies for improving their repair. J Exp Orthop. 2016;3:15. https://doi.org/10.1186/s40634-016-0051-7
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.